Molecular diagnostic and predictive tests in the evolution of chronic hepatitis C anti-viral therapies
- PMID: 23173776
- PMCID: PMC3495636
- DOI: 10.1186/1471-2334-12-S2-S8
Molecular diagnostic and predictive tests in the evolution of chronic hepatitis C anti-viral therapies
Abstract
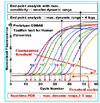
Since the discovery of HCV, polymerase chain reaction (PCR) has significantly contributed to the understanding of the virus life cycle and its replicative kinetics during anti-viral therapy. Parallel to the progression of dual and triple combination treatment, real-time PCR molecular tests have constantly improved in their ability to monitor viral load and drive personalized management schedules. The current sensitivity, accuracy and dynamic range of the available assays fulfil the requirement of "companion diagnostics" and support the development of new directly acting antiviral (DAA)-based regimens.
Figures

Similar articles
-
Molecular diagnostics in the management of chronic hepatitis C: key considerations in the era of new antiviral therapies.BMC Infect Dis. 2014;14 Suppl 5(Suppl 5):S8. doi: 10.1186/1471-2334-14-S5-S8. Epub 2014 Sep 5. BMC Infect Dis. 2014. PMID: 25236936 Free PMC article. Review.
-
Comparison of on-treatment HCV RNA during direct antiviral therapy using two different COBAS TaqMan HCV assays.J Clin Virol. 2017 Apr;89:51-56. doi: 10.1016/j.jcv.2017.02.008. Epub 2017 Feb 24. J Clin Virol. 2017. PMID: 28259054
-
Clinical Laboratory Testing in the Era of Directly Acting Antiviral Therapies for Hepatitis C.Clin Microbiol Rev. 2017 Jan;30(1):23-42. doi: 10.1128/CMR.00037-16. Epub 2016 Oct 19. Clin Microbiol Rev. 2017. PMID: 27795306 Free PMC article. Review.
-
Evaluation of the cobas® GT hepatitis C virus genotyping assay in G1-6 viruses including low viral loads and LiPA failures.PLoS One. 2018 Mar 22;13(3):e0194396. doi: 10.1371/journal.pone.0194396. eCollection 2018. PLoS One. 2018. PMID: 29566005 Free PMC article.
-
Clinical performances of two real-time PCR assays and bDNA/TMA to early monitor treatment outcome in patients with chronic hepatitis C.J Clin Virol. 2009 Nov;46(3):216-21. doi: 10.1016/j.jcv.2009.08.011. Epub 2009 Sep 12. J Clin Virol. 2009. PMID: 19748822
Cited by
-
Towards HCV extinction with modern HCV treatment? "Yes we can!".BMC Infect Dis. 2012;12 Suppl 2(Suppl 2):S1. doi: 10.1186/1471-2334-12-S2-S1. Epub 2012 Nov 12. BMC Infect Dis. 2012. PMID: 23174030 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

