Induction of cross-protection against influenza A virus by DNA prime-intranasal protein boost strategy based on nucleoprotein
- PMID: 23173785
- PMCID: PMC3511278
- DOI: 10.1186/1743-422X-9-286
Induction of cross-protection against influenza A virus by DNA prime-intranasal protein boost strategy based on nucleoprotein
Abstract
Background: The highly conserved nucleoprotein (NP) is an internal protein of influenza virus and is capable of inducing cross-protective immunity against different influenza A viruses, making it a main target of universal influenza vaccine. In current study, we characterized the immune response induced by DNA prime-intranasal protein boost strategy based on NP (A/PR/8/34, H1N1) in mouse model, and evaluated its protection ability against a lethal dose challenge of influenza virus.
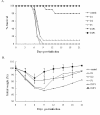
Results: The intranasal boost with recombinant NP (rNP) protein could effectively enhance the pre-immune response induced by the NP DNA vaccine in mice. Compared to the vaccination with NP DNA or rNP protein alone, the prime-boost strategy increased the level of NP specific serum antibody, enhanced the T cell immune response, and relatively induced more mucosal IgA antibody. The overall immune response induced by this heterologous prime-boost regimen was Th-1-biased. Furthermore, the immune response in mice induced by this strategy provided not only protection against the homologous virus but also cross-protection against a heterosubtypic H9N2 strain.
Conclusions: The NP DNA prime-intranasal protein boost strategy may provide an effective strategy for universal influenza vaccine development.
Figures
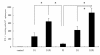


Similar articles
-
Cross-protection against influenza virus infection by intranasal administration of nucleoprotein-based vaccine with compound 48/80 adjuvant.Hum Vaccin Immunother. 2015;11(2):397-406. doi: 10.4161/21645515.2014.995056. Hum Vaccin Immunother. 2015. PMID: 25607884 Free PMC article.
-
A Bivalent Heterologous DNA Virus-Like-Particle Prime-Boost Vaccine Elicits Broad Protection against both Group 1 and 2 Influenza A Viruses.J Virol. 2017 Apr 13;91(9):e02052-16. doi: 10.1128/JVI.02052-16. Print 2017 May 1. J Virol. 2017. PMID: 28179535 Free PMC article.
-
Protein transduction domain-mediated influenza NP subunit vaccine generates a potent immune response and protection against influenza virus in mice.Emerg Microbes Infect. 2020 Dec;9(1):1933-1942. doi: 10.1080/22221751.2020.1812436. Emerg Microbes Infect. 2020. PMID: 32811334 Free PMC article.
-
Harnessing Local Immunity for an Effective Universal Swine Influenza Vaccine.Viruses. 2017 May 5;9(5):98. doi: 10.3390/v9050098. Viruses. 2017. PMID: 28475122 Free PMC article. Review.
-
Potentiating Lung Mucosal Immunity Through Intranasal Vaccination.Front Immunol. 2021 Dec 14;12:808527. doi: 10.3389/fimmu.2021.808527. eCollection 2021. Front Immunol. 2021. PMID: 34970279 Free PMC article. Review.
Cited by
-
Mucosal immunization with integrase-defective lentiviral vectors protects against influenza virus challenge in mice.PLoS One. 2014 May 13;9(5):e97270. doi: 10.1371/journal.pone.0097270. eCollection 2014. PLoS One. 2014. PMID: 24824623 Free PMC article.
-
Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization.Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):10172-10177. doi: 10.1073/pnas.1707950114. Epub 2017 Sep 5. Proc Natl Acad Sci U S A. 2017. PMID: 28874545 Free PMC article.
-
Expression of H3N2 nucleoprotein in maize seeds and immunogenicity in mice.Plant Cell Rep. 2015 Jun;34(6):969-80. doi: 10.1007/s00299-015-1758-0. Epub 2015 Feb 13. Plant Cell Rep. 2015. PMID: 25677970
-
Enhancing Immune Response and Heterosubtypic Protection Ability of Inactivated H7N9 Vaccine by Using STING Agonist as a Mucosal Adjuvant.Front Immunol. 2019 Sep 27;10:2274. doi: 10.3389/fimmu.2019.02274. eCollection 2019. Front Immunol. 2019. PMID: 31611875 Free PMC article.
-
M2e-Based Influenza Vaccines with Nucleoprotein: A Review.Vaccines (Basel). 2021 Jul 4;9(7):739. doi: 10.3390/vaccines9070739. Vaccines (Basel). 2021. PMID: 34358155 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

