Blocking proinflammatory cytokine release modulates peripheral blood mononuclear cell response to Porphyromonas gingivalis
- PMID: 23173823
- PMCID: PMC3935330
- DOI: 10.1902/jop.2012.120422
Blocking proinflammatory cytokine release modulates peripheral blood mononuclear cell response to Porphyromonas gingivalis
Abstract
Background: Chronic periodontitis (CP) is an inflammatory disease in which cytokines play a major role in the progression of disease. Anti-inflammatory cytokines (interleukin 4 [IL-4] and IL-10) were reported to be absent or reduced in diseased periodontal tissues, suggesting an imbalance between the proinflammatory and anti-inflammatory mediators. This study tests the hypothesis that there is cellular crosstalk mediated by proinflammatory and anti-inflammatory cytokines and that blocking proinflammatory cytokine (tumor necrosis factor-α [TNF-α] and IL-1) production will enhance anti-inflammatory cytokine (IL-4 and IL-10) production from peripheral blood mononuclear cells (PBMCs) in response to Porphyromonas gingivalis.
Methods: PBMCs were isolated from individuals diagnosed with CP or healthy individuals and cultured for 24 hours. Concanavalin A (ConA) was used as an activator of lymphocyte function. Live and heat-killed P. gingivalis or lipopolysaccharide from P. gingivalis were used as the bacterial stimulants. TNF-α and IL-1 production was neutralized by specific antibodies against TNF-α and IL-1α or IL-β. Culture supernatants were evaluated by enzyme-linked immunosorbent assay for TNF-α, IL-1β, IL-4, and IL-10 production.
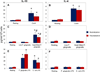
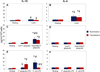
Results: Live P. gingivalis did not result in any significant IL-10 or IL-4 release, whereas heat-killed P. gingivalis led to a significant increase in IL-10 levels compared with unstimulated or live P. gingivalis-stimulated cells from both healthy individuals or those with CP. Overall, PBMCs from patients with CP produced significantly lower IL-10 in response to ConA and P. gingivalis, suggesting chronic suppression of the anti-inflammatory cytokine production. Blocking the proinflammatory cytokine response did not result in any substantial change in IL-10 or IL-4 response to live P. gingivalis. Blocking the proinflammatory cytokine response restored IL-10 production by cells from CP in response to P. gingivalis lipopolysaccharide.
Conclusions: These findings suggest that PBMCs from patients with CP have suppressed anti-inflammatory cytokine production that can, in part, be restored by neutralizing proinflammatory cytokines. Monocytes are an important source of IL-10 production, and monocyte-derived IL-10 might play a regulatory role in the pathogenesis of CP.
Conflict of interest statement
None of the authors have any conflict of interest.
Figures

Similar articles
-
An Explorative Study on Monocyte Reprogramming in the Context of Periodontitis In Vitro and In Vivo.Front Immunol. 2021 Aug 13;12:695227. doi: 10.3389/fimmu.2021.695227. eCollection 2021. Front Immunol. 2021. PMID: 34484192 Free PMC article.
-
Interleukin-1alpha stimulation in monocytes by periodontal bacteria: antagonistic effects of Porphyromonas gingivalis.Oral Microbiol Immunol. 2007 Feb;22(1):52-60. doi: 10.1111/j.1399-302X.2007.00322.x. Oral Microbiol Immunol. 2007. PMID: 17241171
-
Induction of interleukin (IL)-1β, IL-10, IL-8 and immunoglobulin G by Porphyromonas gingivalis HmuY in humans.J Periodontal Res. 2012 Feb;47(1):27-32. doi: 10.1111/j.1600-0765.2011.01401.x. Epub 2011 Aug 17. J Periodontal Res. 2012. PMID: 21848614
-
Subgingival microbial profile and production of proinflammatory cytokines in chronic periodontitis.Folia Med (Plovdiv). 2014 Jul-Sep;56(3):152-60. doi: 10.2478/folmed-2014-0022. Folia Med (Plovdiv). 2014. PMID: 25434071 Review.
-
Role of Porphyromonas gingivalis HmuY in Immunopathogenesis of Chronic Periodontitis.Mediators Inflamm. 2016;2016:7465852. doi: 10.1155/2016/7465852. Epub 2016 Jun 15. Mediators Inflamm. 2016. PMID: 27403039 Free PMC article. Review.
Cited by
-
Oral Biofilm Composition, Dissemination to Keratinocytes, and Inflammatory Attenuation Depend on Probiotic and Synbiotic Strain Specificity.Probiotics Antimicrob Proteins. 2024 Apr 15. doi: 10.1007/s12602-024-10253-z. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 38619794
-
Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in reponse to Porphyromonas gingivalis LPS.Mediators Inflamm. 2013;2013:407562. doi: 10.1155/2013/407562. Epub 2013 Dec 2. Mediators Inflamm. 2013. PMID: 24363500 Free PMC article.
-
Arginine-specific gingipains (RgpA/RgpB) knockdown modulates neutrophil machinery.J Oral Microbiol. 2024 Jul 9;16(1):2376462. doi: 10.1080/20002297.2024.2376462. eCollection 2024. J Oral Microbiol. 2024. PMID: 38988325 Free PMC article.
-
Investigating the biological properties of carbohydrate derived fulvic acid (CHD-FA) as a potential novel therapy for the management of oral biofilm infections.BMC Oral Health. 2013 Sep 24;13:47. doi: 10.1186/1472-6831-13-47. BMC Oral Health. 2013. PMID: 24063298 Free PMC article.
-
Association between Periodontal Health Status and Cognitive Abilities. The Role of Cytokine Profile and Systemic Inflammation.Curr Alzheimer Res. 2017;14(9):978-990. doi: 10.2174/1567205014666170316163340. Curr Alzheimer Res. 2017. PMID: 28317488 Free PMC article.
References
-
- Grenier D, La VD. Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: a review article. Curr Drug Targets. 2011;12:322–331. - PubMed
-
- Lin L, Li C, Liu J, et al. Virulence genes of Porphyromonas gingivalis W83 in chronic periodontitis. Acta Odontol Scand. 2009;67:258–264. - PubMed
-
- Huang PA, Roth GA, Cheng B, et al. Enhanced monocyte migration and pro-inflammatory cytokine production by Porphyromonas gingivalis infection. J Periodont Res. 2010;45:239–245. - PubMed
-
- Wang PL, Ohura K. 2002. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and toll-like receptors. Crit Rev Oral Med. 2002;13:132–142. - PubMed
-
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontology 2000. 1997;14:33–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

