Cannabidiol (CBD) enhances lipopolysaccharide (LPS)-induced pulmonary inflammation in C57BL/6 mice
- PMID: 23173851
- PMCID: PMC3632657
- DOI: 10.3109/1547691X.2012.741628
Cannabidiol (CBD) enhances lipopolysaccharide (LPS)-induced pulmonary inflammation in C57BL/6 mice
Abstract
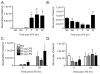
Cannabidiol (CBD) is a plant-derived cannabinoid that has been predominantly characterized as anti-inflammatory. However, it is clear that immune effects of cannabinoids can vary with cannabinoid concentration, or type or magnitude of immune stimulus. The present studies demonstrate that oral administration of CBD enhanced lipopolysaccharide (LPS)-induced pulmonary inflammation in C57BL/6 mice. The enhanced inflammatory cell infiltrate as observed in bronchoalveolar lavage fluid (BALF) was comprised mainly of neutrophils, with some monocytes. Concomitantly, CBD enhanced pro-inflammatory cytokine mRNA production, including tumor necrosis factor-α (Tnfa), interleukins (IL)-5 and -23 (Il6, Il23), and granulocyte colony stimulating factor (Gcsf). These results demonstrate that the CBD-mediated enhancement of LPS-induced pulmonary inflammation is mediated at the level of transcription of a variety of pro-inflammatory genes. The significance of these studies is that CBD is part of a therapeutic currently in use for spasticity and pain in multiple sclerosis patients, and therefore it is important to further understand mechanisms by which CBD alters immune function.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Figures
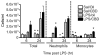


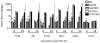
Similar articles
-
Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury.Immunopharmacol Immunotoxicol. 2015 Feb;37(1):35-41. doi: 10.3109/08923973.2014.976794. Epub 2014 Oct 30. Immunopharmacol Immunotoxicol. 2015. PMID: 25356537
-
Effects of alpha 1-antitrypsin on endotoxin-induced lung inflammation in vivo.Inflamm Res. 2010 Jul;59(7):571-8. doi: 10.1007/s00011-010-0164-x. Epub 2010 Mar 18. Inflamm Res. 2010. PMID: 20238140
-
Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells.J Biol Chem. 2010 Jan 15;285(3):1616-26. doi: 10.1074/jbc.M109.069294. Epub 2009 Nov 12. J Biol Chem. 2010. PMID: 19910459 Free PMC article.
-
A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment.Molecules. 2020 Oct 25;25(21):4930. doi: 10.3390/molecules25214930. Molecules. 2020. PMID: 33113776 Free PMC article. Review.
-
Modulatory Potential of Cannabidiol on the Opioid-Induced Inflammatory Response.Cannabis Cannabinoid Res. 2021 Jun;6(3):211-220. doi: 10.1089/can.2020.0181. Cannabis Cannabinoid Res. 2021. PMID: 34115948 Free PMC article. Review.
Cited by
-
Cannabinoids and Inflammations of the Gut-Lung-Skin Barrier.J Pers Med. 2021 May 31;11(6):494. doi: 10.3390/jpm11060494. J Pers Med. 2021. PMID: 34072930 Free PMC article. Review.
-
Cannabis use, oral dysbiosis, and neurological disorders.NeuroImmune Pharm Ther. 2024 Aug 9;3(3-4):183-193. doi: 10.1515/nipt-2024-0012. eCollection 2024 Sep. NeuroImmune Pharm Ther. 2024. PMID: 39741560 Free PMC article. Review.
-
Immune Responses Regulated by Cannabidiol.Cannabis Cannabinoid Res. 2020 Feb 27;5(1):12-31. doi: 10.1089/can.2018.0073. eCollection 2020 Mar 1. Cannabis Cannabinoid Res. 2020. PMID: 32322673 Free PMC article. Review.
-
Is cannabidiol a drug acting on unconventional targets to control drug-resistant epilepsy?Epilepsia Open. 2020 Jan 17;5(1):36-49. doi: 10.1002/epi4.12376. eCollection 2020 Mar. Epilepsia Open. 2020. PMID: 32140642 Free PMC article. Review.
-
Cannabis, Cannabinoids, and the Endocannabinoid System-Is there Therapeutic Potential for Inflammatory Bowel Disease?J Crohns Colitis. 2019 Mar 30;13(4):525-535. doi: 10.1093/ecco-jcc/jjy185. J Crohns Colitis. 2019. PMID: 30418525 Free PMC article. Review.
References
-
- Agurell S, Carlsson S, Lindgren JE, Ohlsson A, Gillespie H, Hollister L. Interactions of Δ1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia. 1981;37:1090–1092. - PubMed
-
- Alm AS, Li K, Chen H, Wang D, Andersson R, Wang X. Variation of lipopolysaccharide-induced acute lung injury in eight strains of mice. Respir Physiol Neurobiol. 2010a;171:157–164. - PubMed
-
- Alm AS, Li K, Yang D, Andersson R, Lu Y, Wang X. Varying susceptibility of pulmonary cytokine production to lipopolysaccharide in mice. Cytokine. 2010b;49:256–263. - PubMed
-
- Altinsoy A, Dilekoz E, Kul O, Ilhan SO, Tunccan OG, Seven I, Bagriacik EU, Sarioglu Y, Or M, Ercan ZS. A cannabinoid ligand, anandamide, exacerbates endotoxin-induced uveitis in rabbits. J Ocular Pharmacol Ther. 2011;27:545–552. - PubMed
-
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: Effect of varying Δ9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
