IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation
- PMID: 23174018
- PMCID: PMC3532073
- DOI: 10.1186/1476-4598-11-87
IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation
Abstract
Background: IL-1β is a pleiotropic pro-inflammatory cytokine and its up-regulation is closely associated with various cancers including gastrointestinal tumors. However, it remains unclear how IL-1β may contribute to the initiation and development of these inflammation-associated cancers. Here we investigated the role of IL-1β in colon cancer stem cell (CSC) development.
Methods: Using self-renewal assay, soft-agar assay, invasion assay, real-time PCR analysis, immunoblot assay and shRNA knockdown, we determined the effects of IL-1β on cancer stem cell development and epithelial-mesenchymal transition (EMT) in human primary colon cancer cells and colon cancer cell line HCT-116.
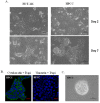
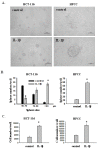
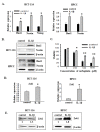
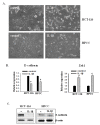
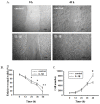
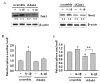
Results: We found that IL-1β can increase sphere-forming capability of colon cancer cells in serum-free medium. IL-1β-induced spheres displayed an up-regulation of stemness factor genes (Bmi1 and Nestin) and increased drug resistance, hallmarks of CSCs. Importantly, expression of EMT activator Zeb1 was increased in IL-1β-induced spheres, indicating that there might be a close association between EMT and IL-1β-induced CSC self-renewal. Indeed, IL-1β treatment led to EMT of colon cancer cells with loss of E-cadherin, up-regulation of Zeb1, and gain of the mesenchymal phenotype. Furthermore, shRNA-mediated knockdown of Zeb1 in HCT-116 cells reversed IL-1β-induced EMT and stem cell formation.
Conclusion: Our findings indicate that IL-1β may promote colon tumor growth and invasion through activation of CSC self-renewal and EMT, and Zeb1 plays a critical role in these two processes. Thus, IL-1β and Zeb1 might be new therapeutic targets against colon cancer stem cells.
Figures

Similar articles
-
EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition.Cancer Biol Ther. 2015;16(6):933-40. doi: 10.1080/15384047.2015.1040959. Epub 2015 Apr 21. Cancer Biol Ther. 2015. PMID: 25897987 Free PMC article.
-
BMP-2 induces motility and invasiveness by promoting colon cancer stemness through STAT3 activation.Tumour Biol. 2015 Dec;36(12):9475-86. doi: 10.1007/s13277-015-3681-y. Epub 2015 Jun 30. Tumour Biol. 2015. PMID: 26124007
-
Epithelial-mesenchymal transition transcription factor ZEB1/ZEB2 co-expression predicts poor prognosis and maintains tumor-initiating properties in head and neck cancer.Oral Oncol. 2013 Jan;49(1):34-41. doi: 10.1016/j.oraloncology.2012.07.012. Epub 2012 Aug 11. Oral Oncol. 2013. PMID: 22892238
-
Role of T-box genes in cancer, epithelial-mesenchymal transition, and cancer stem cells.J Cell Biochem. 2022 Feb;123(2):215-230. doi: 10.1002/jcb.30188. Epub 2021 Dec 13. J Cell Biochem. 2022. PMID: 34897787 Review.
-
The Transcription Factors Zeb1 and Snail Induce Cell Malignancy and Cancer Stem Cell Phenotype in Prostate Cells, Increasing Androgen Synthesis Capacity and Therapy Resistance.Adv Exp Med Biol. 2022;1393:51-64. doi: 10.1007/978-3-031-12974-2_2. Adv Exp Med Biol. 2022. PMID: 36587301 Review.
Cited by
-
Clinical significance of serum interleukin-29, interleukin-32, and tumor necrosis factor alpha levels in patients with gastric cancer.Tumour Biol. 2016 Jan;37(1):405-12. doi: 10.1007/s13277-015-3829-9. Epub 2015 Jul 29. Tumour Biol. 2016. PMID: 26219901
-
Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice.Gastroenterology. 2015 Dec;149(7):1884-1895.e4. doi: 10.1053/j.gastro.2015.07.064. Epub 2015 Aug 7. Gastroenterology. 2015. PMID: 26261008 Free PMC article.
-
Visualization of epithelial-mesenchymal transition in an inflammatory microenvironment-colorectal cancer network.Sci Rep. 2019 Nov 8;9(1):16378. doi: 10.1038/s41598-019-52816-z. Sci Rep. 2019. PMID: 31705021 Free PMC article.
-
Epigenetic Regulation of Inflammatory Cytokine-Induced Epithelial-To-Mesenchymal Cell Transition and Cancer Stem Cell Generation.Cells. 2019 Sep 25;8(10):1143. doi: 10.3390/cells8101143. Cells. 2019. PMID: 31557902 Free PMC article. Review.
-
UBE2C, Directly Targeted by miR-548e-5p, Increases the Cellular Growth and Invasive Abilities of Cancer Cells Interacting with the EMT Marker Protein Zinc Finger E-box Binding Homeobox 1/2 in NSCLC.Theranostics. 2019 Mar 17;9(7):2036-2055. doi: 10.7150/thno.32738. eCollection 2019. Theranostics. 2019. Retraction in: Theranostics. 2020 Jul 25;10(21):9619. doi: 10.7150/thno.50254. PMID: 31037155 Free PMC article. Retracted.
References
-
- Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

