Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch
- PMID: 23174199
- PMCID: PMC4610374
- DOI: 10.1016/j.vaccine.2012.11.027
Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch
Abstract
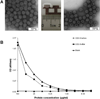
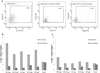
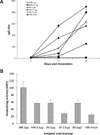
Skin immunization is effective against a number of infectious diseases, including smallpox and tuberculosis, but is difficult to administer. Here, we assessed the use of an easy-to-administer microneedle (MN) patch for skin vaccination using an inactivated rotavirus vaccine (IRV) in mice. Female inbred BALB/c mice in groups of six were immunized once in the skin using MN coated with 5 μg or 0.5 μg of inactivated rotavirus antigen or by intramuscular (IM) injection with 5 μg or 0.5 μg of the same antigen, bled at 0 and 10 days, and exsanguinated at 28 days. Rotavirus-specific IgG titers increased over time in sera of mice immunized with IRV using MN or IM injection. However, titers of IgG and neutralizing activity were generally higher in MN immunized mice than in IM immunized mice; the titers in mice that received 0.5 μg of antigen with MN were comparable or higher than those that received 5 μg of antigen IM, indicating dose sparing. None of the mice receiving negative-control, antigen-free MN had any IgG titers. In addition, MN immunization was at least as effective as IM administration in inducing a memory response of dendritic cells in the spleen. Our findings demonstrate that MN delivery can reduce the IRV dose needed to mount a robust immune response compared to IM injection and holds promise as a strategy for developing a safer and more effective rotavirus vaccine for use among children throughout the world.
Keywords: IRV; Microneedle; Rotavirus; Skin immunization.
Published by Elsevier Ltd.
Conflict of interest statement
Figures

Similar articles
-
Lack of immune interference between inactivated polio vaccine and inactivated rotavirus vaccine co-administered by intramuscular injection in two animal species.Vaccine. 2019 Jan 29;37(5):698-704. doi: 10.1016/j.vaccine.2018.12.043. Epub 2019 Jan 6. Vaccine. 2019. PMID: 30626530
-
Skin Vaccination against Rotavirus Using Microneedles: Proof of Concept in Gnotobiotic Piglets.PLoS One. 2016 Nov 8;11(11):e0166038. doi: 10.1371/journal.pone.0166038. eCollection 2016. PLoS One. 2016. PMID: 27824918 Free PMC article.
-
Inactivated rotavirus vaccine by parenteral administration induces mucosal immunity in mice.Sci Rep. 2018 Jan 12;8(1):561. doi: 10.1038/s41598-017-18973-9. Sci Rep. 2018. PMID: 29330512 Free PMC article.
-
Microneedle-based vaccines.Curr Top Microbiol Immunol. 2009;333:369-93. doi: 10.1007/978-3-540-92165-3_18. Curr Top Microbiol Immunol. 2009. PMID: 19768415 Free PMC article. Review.
-
Inactivated rotavirus vaccines: a priority for accelerated vaccine development.Vaccine. 2008 Dec 9;26(52):6754-8. doi: 10.1016/j.vaccine.2008.10.008. Epub 2008 Oct 23. Vaccine. 2008. PMID: 18951937 Review.
Cited by
-
Needle-Free Immunization with Chitosan-Based Systems.Int J Mol Sci. 2018 Nov 19;19(11):3639. doi: 10.3390/ijms19113639. Int J Mol Sci. 2018. PMID: 30463211 Free PMC article. Review.
-
The potential role of using vaccine patches to induce immunity: platform and pathways to innovation and commercialization.Expert Rev Vaccines. 2020 Feb;19(2):175-194. doi: 10.1080/14760584.2020.1732215. Epub 2020 Mar 17. Expert Rev Vaccines. 2020. PMID: 32182145 Free PMC article. Review.
-
Skin permeabilization for transdermal drug delivery: recent advances and future prospects.Expert Opin Drug Deliv. 2014 Mar;11(3):393-407. doi: 10.1517/17425247.2014.875528. Epub 2014 Jan 7. Expert Opin Drug Deliv. 2014. PMID: 24392787 Free PMC article. Review.
-
Vaccines for viral and bacterial pathogens causing acute gastroenteritis: Part I: Overview, vaccines for enteric viruses and Vibrio cholerae.Hum Vaccin Immunother. 2015;11(3):584-600. doi: 10.1080/21645515.2015.1011019. Hum Vaccin Immunother. 2015. PMID: 25715048 Free PMC article. Review.
-
The performance of licensed rotavirus vaccines and the development of a new generation of rotavirus vaccines: a review.Hum Vaccin Immunother. 2021 Mar 4;17(3):880-896. doi: 10.1080/21645515.2020.1801071. Epub 2020 Sep 23. Hum Vaccin Immunother. 2021. PMID: 32966134 Free PMC article. Review.
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. - PubMed
-
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. - PubMed
-
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human–bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. [see comment] - PubMed
-
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. - PubMed
-
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

