Inflammatory T helper 17 cells promote depression-like behavior in mice
- PMID: 23174342
- PMCID: PMC3582833
- DOI: 10.1016/j.biopsych.2012.09.021
Inflammatory T helper 17 cells promote depression-like behavior in mice
Abstract
Background: Recognition of substantial immune-neural interactions is revising dogmas about their insular actions and revealing that immune-neural interactions can substantially impact central nervous system functions. The inflammatory cytokine interleukin-6 promotes susceptibility to depression and drives production of inflammatory T helper 17 (Th17) T cells, raising the hypothesis that in mouse models, Th17 cells promote susceptibility to depression-like behaviors.
Methods: Behavioral characteristics were measured in male mice administered Th17 cells, CD4(+) cells, or vehicle and in retinoid-related orphan receptor-γT (RORγT)(+/GFP) mice or male mice treated with RORγT inhibitor or anti-interleukin-17A antibodies.
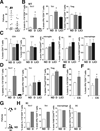
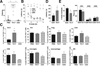
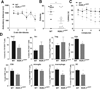
Results: Mouse brain Th17 cells were elevated by learned helplessness and chronic restraint stress, two common depression-like models. Th17 cell administration promoted learned helplessness in 89% of mice in a paradigm where no vehicle-treated mice developed learned helplessness, and impaired novelty suppressed feeding and social interaction behaviors. Mice deficient in the RORγT transcription factor necessary for Th17 cell production exhibited resistance to learned helplessness, identifying modulation of RORγT as a potential intervention. Treatment with the RORγT inhibitor SR1001, or anti-interleukin-17A antibodies to abrogate Th17 cell function, reduced Th17-dependent learned helplessness.
Conclusions: These findings indicate that Th17 cells are increased in the brain during depression-like states, promote depression-like behaviors in mice, and specifically inhibiting the production or function of Th17 cells reduces vulnerability to depression-like behavior, suggesting antidepressant effects may be attained by targeting Th17 cells.
Copyright © 2013 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures

Comment in
-
Distinct characteristics of hippocampal pathogenic TH17 cells in a mouse model of depression.Brain Behav Immun. 2018 Oct;73:180-191. doi: 10.1016/j.bbi.2018.04.012. Epub 2018 Apr 24. Brain Behav Immun. 2018. PMID: 29698707 Free PMC article.
References
-
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. - PubMed
-
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. - PubMed
-
- Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

