An updated meta-analysis to understand the variable efficacy of drotrecogin alfa (activated) in severe sepsis and septic shock
- PMID: 23174922
- PMCID: PMC3725305
An updated meta-analysis to understand the variable efficacy of drotrecogin alfa (activated) in severe sepsis and septic shock
Abstract
Background: Significant debate continues over the efficacy of drotrecogin alpha activated (DAA) in sepsis. This updated meta-analysis provides an updated summary effect estimate and explores the reasons for outcome heterogeneity in placebo-controlled randomized clinical trials of DAA on 28-day all-cause mortality in patients with severe sepsis or septic shock.
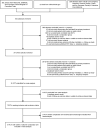
Methods: Computer searches of MEDLINE, EMBASE, the Cochrane Library, ClinicalTrials.gov, published abstracts from major intensive care meetings and examination of reference lists were used to identify five placebo-controlled randomized clinical trials with 7260 patients. The primary endpoint was 28-day all-cause mortality. Secondary outcomes were 28-day incidence of severe bleeding and intracranial hemorrhage.
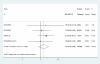
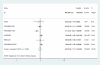
Results: DAA was not associated with improved 28-day all-cause mortality in patients with severe sepsis or septic shock (pooled relative risk (RR) of 0.97 [95% CI 0.83-1.14]), and is associated with an increase in serious bleeding. The significant heterogeneity in the pooled RR for 28-day mortality (I2 value of 59.4%, χ2 P-value 0.043) is no longer present with exclusion of the post-study amendment portion of PROWESS (I2 value of 0%, χ2 P-value 0.44 without PROWESS post-amendment). Using meta-regression, the best ranked predictor of outcome heterogeneity was baseline mortality in the placebo arm, which was among the highest in PROWESS.
Conclusion: DAA is not associated with improved survival in patients with severe sepsis or septic shock. Further studies should be done to determine whether changes in supportive therapy for sepsis explain the variable efficacy of DAA in randomized controlled clinical trials observed over time.
Figures



Comment in
-
We've made progress in the treatment of sepsis, so do we still need sepsis trials?Minerva Anestesiol. 2013 Jan;79(1):1-2. Minerva Anestesiol. 2013. PMID: 23299044 No abstract available.
Similar articles
-
Drotrecogin alfa (recombinant human activated protein C) for the treatment of severe sepsis.Clin Ther. 2003 Feb;25(2):396-421. doi: 10.1016/s0149-2918(03)80086-3. Clin Ther. 2003. PMID: 12749504 Review.
-
Extended evaluation of recombinant human activated protein C United States Trial (ENHANCE US): a single-arm, phase 3B, multicenter study of drotrecogin alfa (activated) in severe sepsis.Chest. 2004 Jun;125(6):2206-16. doi: 10.1378/chest.125.6.2206. Chest. 2004. PMID: 15189943 Clinical Trial.
-
Drotrecogin alfa (activated) administration across clinically important subgroups of patients with severe sepsis.Crit Care Med. 2003 Jan;31(1):12-9. doi: 10.1097/00003246-200301000-00002. Crit Care Med. 2003. PMID: 12544987 Clinical Trial.
-
Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression.Lancet Infect Dis. 2012 Sep;12(9):678-86. doi: 10.1016/S1473-3099(12)70157-3. Epub 2012 Jul 17. Lancet Infect Dis. 2012. PMID: 22809883
-
Drotrecogin alfa (activated): a pharmacoeconomic review of its use in severe sepsis.Pharmacoeconomics. 2004;22(7):445-76. doi: 10.2165/00019053-200422070-00004. Pharmacoeconomics. 2004. PMID: 15137883 Review.
Cited by
-
A Dormant Microbial Component in the Development of Preeclampsia.Front Med (Lausanne). 2016 Nov 29;3:60. doi: 10.3389/fmed.2016.00060. eCollection 2016. Front Med (Lausanne). 2016. PMID: 27965958 Free PMC article. Review.
-
Recombinant human activated protein C for the treatment of severe sepsis and septic shock: a study protocol for incorporating observational evidence using a Bayesian approach.BMJ Open. 2014 Jul 31;4(7):e005622. doi: 10.1136/bmjopen-2014-005622. BMJ Open. 2014. PMID: 25082420 Free PMC article. Review.
-
[Lost in translation? On the effectiveness and efficacy of drotrecogin alfa (recombinant human activated protein C)].Med Klin Intensivmed Notfmed. 2013 Feb;108(1):69-70. doi: 10.1007/s00063-012-0206-y. Med Klin Intensivmed Notfmed. 2013. PMID: 23263456 German. No abstract available.
-
The Emperor Has No Clothes? Searching for Dysregulation in Sepsis.J Clin Med. 2018 Aug 29;7(9):247. doi: 10.3390/jcm7090247. J Clin Med. 2018. PMID: 30158480 Free PMC article. Review.
-
Flow Cytometry-Based Quantification of Neutrophil Extracellular Traps Shows an Association with Hypercoagulation in Septic Shock and Hypocoagulation in Postsurgical Systemic Inflammation-A Proof-of-Concept Study.J Clin Med. 2020 Jan 8;9(1):174. doi: 10.3390/jcm9010174. J Clin Med. 2020. PMID: 31936385 Free PMC article.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. - PubMed
-
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–53. - PubMed
-
- Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–72. - PubMed
-
- Russell JA. Management of sepsis. N Engl J Med. 2006;355(16):1699–713. - PubMed
-
- Ferrer R, Artigas A, Levy MM, Blanco J, Gonzalez-Diaz G, Garnacho-Montero J, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

