Reaction-based small-molecule fluorescent probes for chemoselective bioimaging
- PMID: 23174976
- PMCID: PMC4096518
- DOI: 10.1038/nchem.1500
Reaction-based small-molecule fluorescent probes for chemoselective bioimaging
Abstract
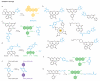
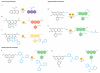
The dynamic chemical diversity of elements, ions and molecules that form the basis of life offers both a challenge and an opportunity for study. Small-molecule fluorescent probes can make use of selective, bioorthogonal chemistries to report on specific analytes in cells and in more complex biological specimens. These probes offer powerful reagents to interrogate the physiology and pathology of reactive chemical species in their native environments with minimal perturbation to living systems. This Review presents a survey of tools and tactics for using such probes to detect biologically important chemical analytes. We highlight design criteria for effective chemical tools for use in biological applications as well as gaps for future exploration.
Figures





References
-
- Czarnik AW. Chemical communication in water using fluorescent chemosensors. Acc. Chem. Res. 1994;27:302–308.
-
- Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008;37:1465–1472. - PubMed
-
- Jun ME, Roy B, Ahn KH. ‘Turn-on’ fluorescent sensing with ‘reactive’ probes. Chem. Commun. 2011;47:7583–7601. - PubMed
-
- Du J, Hu M, Fan J, Peng X. Fluorescent chemodosimeters using ‘mild’ chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012;41:4511–4535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

