Combinatorial evolution of site- and enantioselective catalysts for polyene epoxidation
- PMID: 23174978
- PMCID: PMC3506257
- DOI: 10.1038/nchem.1469
Combinatorial evolution of site- and enantioselective catalysts for polyene epoxidation
Erratum in
- Nat Chem. 2013 Jan;5(1):74
Abstract
Selectivity in the catalytic functionalization of complex molecules is a major challenge in chemical synthesis. The problem is magnified when there are several possible stereochemical outcomes and when similar functional groups occur repeatedly within the same molecule. Selective polyene oxidation provides an archetypical example of this challenge. Historically, enzymatic catalysis has provided the only precedents. Although non-enzymatic catalysts that meet some of these challenges became known, a comprehensive solution has remained elusive. Here, we describe low molecular weight peptide-based catalysts, discovered through a combinatorial synthesis and screening protocol, that exhibit site- and enantioselective oxidation of certain positions of various isoprenols. This diversity-based approach, which exhibits features reminiscent of the directed evolution of enzymes, delivers catalysts that compare favourably to the state-of-the-art for the asymmetric oxidation of these compounds. Moreover, the approach culminated in catalysts that exhibit alternative-site selectivity in comparison to oxidation catalysts previously described.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

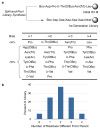

Comment in
-
Site-selective reactions: Remodelling by diversity and design.Nat Chem. 2012 Dec;4(12):963-5. doi: 10.1038/nchem.1509. Nat Chem. 2012. PMID: 23174971 No abstract available.
References
-
- van Tamelen EE, Heys JR. Enzymic epoxidation of squalene variants. J Am Chem Soc. 1974;97:1252–1253. - PubMed
-
- Katsuki T, Martin VS. Asymmetric epoxidation of allylic alcohols: The Katsuki-Sharpless epoxidation reaction. Org React. 1996;48:1–299.
-
- Zhang W, Basak A, Kosugi Y, Hoshino Y, Yamamoto H. Enantioselective epoxidation of allylic alcohols by a chiral complex of vanadium: an effective controller system and a rational mechanistic model. Angew Chem Int Ed. 2005;44:4389–4391. - PubMed
-
- Malkov AV, Czemerys L, Malyshev DA. Vanadium-catalyzed asymmetric epoxidation of allylic alcohols in water. J Org Chem. 2009;74:3350–3355. - PubMed
-
- Egami H, Oguma T, Katsuki T. Oxidation catalysis of Nb(salan) complexes: asymmetric epoxidation of allylic alcohols using aqueous hydrogen peroxide. J Am Chem Soc. 2010;132:5886–5895. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/144187721
- PubChem-Substance/144187722
- PubChem-Substance/144187723
- PubChem-Substance/144187724
- PubChem-Substance/144187725
- PubChem-Substance/144187726
- PubChem-Substance/144187727
- PubChem-Substance/144187728
- PubChem-Substance/144187729
- PubChem-Substance/144187730
- PubChem-Substance/144187731
- PubChem-Substance/144187732
- PubChem-Substance/144187733
Grants and funding
LinkOut - more resources
Full Text Sources

