Evaluation of survival benefits by platinums and taxanes for an unfavourable subset of carcinoma of unknown primary: a systematic review and meta-analysis
- PMID: 23175147
- PMCID: PMC3553519
- DOI: 10.1038/bjc.2012.516
Evaluation of survival benefits by platinums and taxanes for an unfavourable subset of carcinoma of unknown primary: a systematic review and meta-analysis
Abstract
Background: Although chemotherapeutic regimens containing a taxane or platinum agent have been widely recommended for unfavourable carcinoma of unknown primary (CUP), no evidence exists for the superiority of any administered regimens. To date, the efficacy has been mostly assessed in the limited setting of phase II trials, and few attempts have been made to synthesise all available data for survival outcomes.
Methods: Electronic databases were searched from 1980 to 2011. Survival results were combined for each pre-specified category of regimens using a random-effects model, and meta-regression models were used to adjust for heterogeneity in some known prognostic factors.
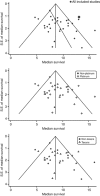
Results: A total of 32 studies were included for meta-analysis. Tendency towards better survival outcome by platinums or taxanes was indicated. After adjustment for important prognostic factors, however, the difference between the platinum-based and non-platinum regimens became no longer significant. Survival benefits by the taxane-based regimens remained significant, with a prolonged median survival time of 1.52 months (P=0.03) and a higher 1-year survival rate of 6.25% (P=0.05), but the benefit did not sustain for 2 years.
Conclusion: Although no effective therapies have been established, this meta-analysis helps to fill an important gap of evidence. However, caution should still be taken because of the potential unmeasured confounding.
Figures



References
-
- Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Lenzi R, Frost P (1994) Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol 12(6): 1272–1280 - PubMed
-
- Adenis A, Ferte C, Penel N (2010) Phase II trials in patients with carcinoma of unknown primary: a pooled data analysis. Invest New Drugs 28(2): 178–184 - PubMed
-
- Assersohn L, Norman AR, Cunningham D, Iveson T, Seymour M, Hickish T, Massey A, Prior Y, Hill ME (2003) A randomised study of protracted venous infusion of 5-fluorouracil (5-FU) with or without bolus mitomycin C (MMC) in patients with carcinoma of unknown primary. Eur J Cancer 39(8): 1121–1128 - PubMed
-
- Balana C, Manzano JL, Moreno I, Cirauqui B, Abad A, Font A, Mate JL, Rosell R (2003) A phase II study of cisplatin, etoposide and gemcitabine in an unfavourable group of patients with carcinoma of unknown primary site. Ann Oncol 14(9): 1425–1429 - PubMed
-
- Briasoulis E, Fountzilas G, Bamias A, Dimopoulos MA, Xiros N, Aravantinos G, Samantas E, Kalofonos H, Makatsoris T, Mylonakis N, Papakostas P, Skarlos D, Varthalitis I, Pavlidis N (2008) Multicenter phase-II trial of irinotecan plus oxaliplatin [IROX regimen] in patients with poor-prognosis cancer of unknown primary: a hellenic cooperative oncology group study. Cancer Chemother Pharmacol 62(2): 277–284 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

