Type-3 metabotropic glutamate receptors regulate chemoresistance in glioma stem cells, and their levels are inversely related to survival in patients with malignant gliomas
- PMID: 23175182
- PMCID: PMC3569992
- DOI: 10.1038/cdd.2012.150
Type-3 metabotropic glutamate receptors regulate chemoresistance in glioma stem cells, and their levels are inversely related to survival in patients with malignant gliomas
Abstract
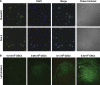
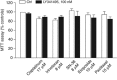
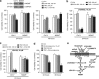
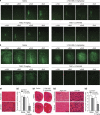
Drug treatment of malignant gliomas is limited by the intrinsic resistance of glioma stem cells (GSCs) to chemotherapy. GSCs isolated from human glioblastoma multiforme (GBM) expressed metabotropic glutamate receptors (mGlu3 receptors). The DNA-alkylating agent, temozolomide, killed GSCs only if mGlu3 receptors were knocked down or pharmacologically inhibited. In contrast, mGlu3 receptor blockade did not affect the action of paclitaxel, etoposide, cis-platinum, and irinotecan. mGlu3 receptor blockade enabled temozolomide toxicity by inhibiting a phosphatidylinositol-3-kinase/nuclear factor-κB pathway that supports the expression of O(6)-methylguanine-DNA methyltransferase (MGMT), an enzyme that confers resistance against DNA-alkylating agents. In mice implanted with GSCs into the brain, temozolomide combined with mGlu3 receptor blockade substantially reduced tumor growth. Finally, 87 patients with GBM undergoing surgery followed by adjuvant chemotherapy with temozolomide survived for longer time if tumor cells expressed low levels of mGlu3 receptors. In addition, the methylation state of the MGMT gene promoter in tumor extracts influenced survival only in those patients with low expression of mGlu3 receptors in the tumor. These data encourage the use of mGlu3 receptor antagonists as add-on drugs in the treatment of GBM, and suggest that the transcript of mGlu3 receptors should be measured in tumor specimens for a correct prediction of patients' survival in response to temozolomide treatment.
Figures




References
-
- Wen PY, Kesari S. Malignant glioma in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumor initiating cells. Nature. 2004;18:396–401. - PubMed
-
- Vescovi AL, Galli R, Reynolds BA. Brain tumor stem cells. Nat Rev Cancer. 2006;6:425–436. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

