Optimization of a combined human parechovirus-enterovirus real-time reverse transcription-PCR assay and evaluation of a new parechovirus 3-specific assay for cerebrospinal fluid specimen testing
- PMID: 23175256
- PMCID: PMC3553926
- DOI: 10.1128/JCM.01982-12
Optimization of a combined human parechovirus-enterovirus real-time reverse transcription-PCR assay and evaluation of a new parechovirus 3-specific assay for cerebrospinal fluid specimen testing
Abstract
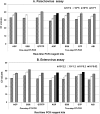
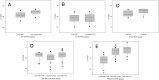
Human parechoviruses (HPeVs), particularly type 3 (HPeV3), are known central nervous system (CNS) pathogens, causing serious infections in infants similar to those caused by enteroviruses (EVs). The primary aim of this study was to combine and validate HPeV and EV real-time reverse transcription-PCR (RT-PCR) detection assays with the best available RT-PCR reagents and conditions for parallel detection of HPeV and EV on a single platform. The secondary aim was to develop and validate a newly developed HPeV3-specific real-time RT-PCR assay. Five commercially available RT-PCR kits were evaluated with the pan-HPeV and EV assays in one-step and two-step RT-PCRs. Two-step RT-PCR with the AgPath ID RT-PCR (AGP) kit performed best for both pan-HPeV and EV assays. The pan-HPeV-specific assay performed best with the AGP kit in a one-step RT-PCR. Frozen aliquots of 145 (for HPeV, n = 70; for EV, n = 75) previously characterized cerebrospinal fluid (CSF) specimens were tested by EV-, pan-HPeV-, and HPeV3-specific (HPeV specimens only) assays. The pan-HPeV and EV assays demonstrated 100% analytical sensitivity and specificity compared to historic results, while the HPeV3-specific assay demonstrated 97% sensitivity and 100% specificity. We propose a real-time pan-HPeV, EV two-step RT-PCR algorithm for simultaneous detection of HPeV and EV from CSF specimens on a single platform. The HPeV3-specific one-step RT-PCR assay can be used as a rapid and cost-effective assay to detect and identify HPeV3 in pan-HPeV RT-PCR assay-positive CSF specimens.
Figures
References
-
- King AMQ, Brown F, Christian P, Hovi T, Hypii T, Knowles NJ, Lemon SM, Minor PD, Palmenberg AC, Skern T, Stanway G. 1999. Picornaviridae, p 996 In van Regenmorrel MHV, Fauquet CM, Bishop DHL, Calisher CH, Carsten EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB. (ed), Virus taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses Academic Press, New York, NY
-
- Benschop KSM, Schinkel J, Minnaar RP, Pajkrt D, Sjerberg L, Kraakman HC, Berkhout B, Zaaijer HL, Beld MG, Wolthers KC. 2006. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin. Infect. Dis. 42:204–210 - PubMed
-
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. 2006. Enterovirus surveillance—United States, 1970-2005. MMWR Morb. Mortal. Wkly. Rep. 55:1–20 - PubMed
-
- Selvarangan R, Nzabi M, Selvaraju SB, Ketter P, Carpenter C, Harrison CJ. 2011. Human parechovirus 3 causing sepsis-like illness in children from midwestern United States. Pediatr. Infect. Dis. J. 30:238–242 - PubMed
-
- Wolthers KC, Benschop KS, Schinkel J, Molenkamp R, Bergevoet RM, Spijkerman IJ, Kraakman HC, Pajkrt D. 2008. Human parechoviruses as an important viral cause of sepsis-like illness and meningitis in young children. Clin. Infect. Dis. 47:358–363 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

