Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion
- PMID: 23175357
- PMCID: PMC3554162
- DOI: 10.1128/JVI.00718-12
Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion
Abstract
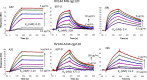
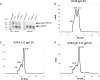
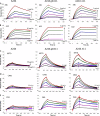
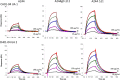
An immune correlates analysis of the RV144 HIV-1 vaccine trial revealed that antibody responses to the gp120 V1/V2 region correlated inversely with infection risk. The RV144 protein immunogens (A244-rp120 and MN-rgp120) were modified by an N-terminal 11-amino-acid deletion (Δ11) and addition of a herpes simplex virus (HSV) gD protein-derived tag (gD). We investigated the effects of these modifications on gp120 expression, antigenicity, and immunogenicity by comparing unmodified A244 gp120 with both Δ11 deletion and gD tag and with Δ11 only. Analysis of A244 gp120, with or without Δ11 or gD, demonstrated that the Δ11 deletion, without the addition of gD, was sufficient for enhanced antigenicity to gp120 C1 region, conformational V2, and V1/V2 gp120 conformational epitopes. RV144 vaccinee serum IgGs bound more avidly to A244 gp120 Δ11 than to the unmodified gp120, and their binding was blocked by C1, V2, and V1/V2 antibodies. Rhesus macaques immunized with the three different forms of A244 gp120 proteins gave similar levels of gp120 antibody titers, although higher antibody titers developed earlier in A244 Δ11 gp120-immunized animals. Conformational V1/V2 monoclonal antibodies (MAbs) gave significantly higher levels of blocking of plasma IgG from A244 Δ11 gp120-immunized animals than IgG from animals immunized with unmodified A244 gp120, thus indicating a qualitative difference in the V1/V2 antibodies induced by A244 Δ11 gp120. These results demonstrate that deletion of N-terminal residues in the RV144 A244 gp120 immunogen improves both envelope antigenicity and immunogenicity.
Figures



References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, Investigators MOPH-TAVEG. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 - PubMed
-
- Berman PW. 1998. Development of bivalent rgp120 vaccines to prevent HIV type 1 infection. AIDS Res. Hum. Retroviruses 14:S277–S289 - PubMed
-
- Berman PW, Huang W, Riddle L, Gray AM, Wrin T, Vennari J, Johnson A, Klaussen M, Prashad H, Kohne deWit CC, Gregory TJ. 1999. Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virology 265:1–9 - PubMed
-
- Francis DP, Gregory T, McElrath MJ, Belshe RB, Gorse GJ, Migasena S, Kitayaporn D, Pitisuttitham P, Matthews T, Schwartz DH, Berman PW. 1998. Advancing AIDSVAX to phase 3: safety, immunogenicity, and plans for phase 3. AIDS Res. Hum. Retroviruses 14:325–331 - PubMed
-
- Lasky LA, Groopman JE, Fennie CW, Benz PM, Capon DJ, Dowbenko DJ, Nakamura GR, Nunes WM, Renz ME, Berman PW. 1986. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science 233:209–212 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

