Sources of variation in flow cytometric analysis of aquatic species sperm: The effect of cryoprotectants on flow cytometry scatter plots and subsequent population gating
- PMID: 23175587
- PMCID: PMC3500965
- DOI: 10.1016/j.aquaculture.2012.09.024
Sources of variation in flow cytometric analysis of aquatic species sperm: The effect of cryoprotectants on flow cytometry scatter plots and subsequent population gating
Abstract
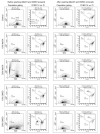
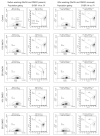
The use of fluorescent staining and flow cytometry to assess sperm quality in aquatic species has increased over the past decade, but comparisons among studies are difficult or impossible due to variation in application, analysis, and reporting of protocols and data.The goal of the present study was to determine the effect of exposure to two cryoprotectants commonly used for cryopreservation of sperm from aquatic species on the accuracy of flow cytometric assessment of sperm quality.Membrane integrity of zebrafish (Danio rerio) sperm exposed to 10% and 20%methanol and dimethyl sulfoxide (DMSO)in 300 mOsm kg(-1) Hanks' balanced salt solution (HBSS) or calcium-free HBSSwas determined using SYBR 14/propidium iodide staining. Both cryoprotectants significantly affected forward-scatter and side-scatter characteristics of sperm samples, resulting in significant changes in the number of total and gated events, and in the number and percentage of intact cells. These results indicate that it cannot be assumed that the approach to flow cytometric analysis of fresh sperm will be applicable to cryoprotectant-treated or cryopreserved sperm. In total, we document examples of five potentially interacting factors that produce errors of 5 to 50% each, resulting in underestimates and overestimates of total and intact sperm (actual numbers and percentages) in the presence of the two most commonly used cryoprotectants at the concentrations used most often for cryopreservation of sperm from aquatic species. This study provides methods to reduce or eliminate these errors and recommendations necessary for standardization and reporting.
Figures

Similar articles
-
Amine reactive dyes: an alternative to estimate membrane integrity in fish sperm cells.Aquaculture. 2016 Oct 1;463:71-78. doi: 10.1016/j.aquaculture.2016.05.031. Epub 2016 May 20. Aquaculture. 2016. PMID: 27818535 Free PMC article.
-
Production of channel catfish with sperm cryopreserved by rapid non-equilibrium cooling.Cryobiology. 2011 Dec;63(3):186-97. doi: 10.1016/j.cryobiol.2011.06.004. Epub 2011 Aug 26. Cryobiology. 2011. PMID: 21896271
-
Determination of sperm concentration using flow cytometry with simultaneous analysis of sperm plasma membrane integrity in zebrafish Danio rerio.Cytometry A. 2016 Apr;89(4):350-6. doi: 10.1002/cyto.a.22796. Epub 2015 Nov 18. Cytometry A. 2016. PMID: 26580311 Free PMC article.
-
A Procedure-Spanning Analysis of Plasma Membrane Integrity for Assessment of Cell Viability in Sperm Cryopreservation of Zebrafish Danio rerio.Zebrafish. 2016 Apr;13(2):144-51. doi: 10.1089/zeb.2015.1176. Epub 2016 Feb 9. Zebrafish. 2016. PMID: 26859531 Free PMC article.
-
Impact of cryoprotectants and cryopreservation on metabolic activity and cytoskeleton proteins of zebrafish (Danio rerio) ovarian fragments.Cryo Letters. 2011 Nov-Dec;32(6):525-36. Cryo Letters. 2011. PMID: 22227713
Cited by
-
Amine reactive dyes: an alternative to estimate membrane integrity in fish sperm cells.Aquaculture. 2016 Oct 1;463:71-78. doi: 10.1016/j.aquaculture.2016.05.031. Epub 2016 May 20. Aquaculture. 2016. PMID: 27818535 Free PMC article.
-
Methyl glycol, methanol and DMSO effects on post-thaw motility, velocities, membrane integrity and mitochondrial function of Brycon orbignyanus and Prochilodus lineatus (Characiformes) sperm.Fish Physiol Biochem. 2015 Feb;41(1):193-201. doi: 10.1007/s10695-014-0016-7. Epub 2014 Nov 30. Fish Physiol Biochem. 2015. PMID: 25433690
-
Immune related biomarkers for cancer metastasis to the brain.Exp Hematol Oncol. 2022 Dec 16;11(1):105. doi: 10.1186/s40164-022-00349-z. Exp Hematol Oncol. 2022. PMID: 36527157 Free PMC article. Review.
-
Addressing Reproducibility in Cryopreservation, and Considerations Necessary for Commercialization and Community Development in Support of Genetic Resources of Aquatic Species.J World Aquac Soc. 2018 Aug;49(4):644-663. doi: 10.1111/jwas.12541. Epub 2018 Jun 28. J World Aquac Soc. 2018. PMID: 30467453 Free PMC article.
-
A quality assurance initiative for commercial-scale production in high-throughput cryopreservation of blue catfish sperm.Cryobiology. 2013 Oct;67(2):214-24. doi: 10.1016/j.cryobiol.2013.07.001. Epub 2013 Jul 18. Cryobiology. 2013. PMID: 23872356 Free PMC article.
References
-
- Alavi SMH, Cosson J. Sperm motility in fishes. (II) Effects of ions and osmolality: a review. Cell. Biol. Int. 2006;30:1–14. - PubMed
-
- Balakin KV, Ivanenkov YA, Skorenko AV, Nikolsky YV, Savchuk NP, Ivashchenko AA. In silico estimation of DMSO solubility of organic compounds for bioscreening. J. Biomol. Screen. 2004;9:22–31. - PubMed
-
- Cabrita E, Robles V, Cuñado S, Wallace JC, Sarasquete C, Herráez MP. Evaluation of gilthead sea bream, Sparus aurata, sperm quality after cryopreservation in 5 ml macrotubes. Cryobiology. 2005;50:273–284. - PubMed
-
- Christensen J, Tiersch T. Cryopreservation of channel catfish sperm: effects of cryoprotectant exposure time, cooling rate, thawing conditions, and male-to-male variation. Theriogenology. 2005;63:2103–2112. - PubMed
-
- Christensen P, Stenvang JP, Godfrey WL. A flow cytometric method for rapid determination of sperm concentration and viability in mammalian and avian semen. J. Androl. 2004;25:255–264. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
