Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis
- PMID: 23175633
- PMCID: PMC3513992
- DOI: 10.1242/dev.086413
Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis
Abstract
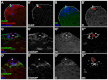
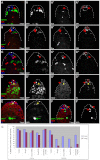
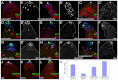
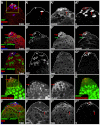
The Drosophila testis harbors two types of stem cells: germ line stem cells (GSCs) and cyst stem cells (CySCs). Both stem cell types share a physical niche called the hub, located at the apical tip of the testis. The niche produces the JAK/STAT ligand Unpaired (Upd) and BMPs to maintain CySCs and GSCs, respectively. However, GSCs also require BMPs produced by CySCs, and as such CySCs are part of the niche for GSCs. Here we describe a role for another secreted ligand, Hedgehog (Hh), produced by niche cells, in the self-renewal of CySCs. Hh signaling cell-autonomously regulates CySC number and maintenance. The Hh and JAK/STAT pathways act independently and non-redundantly in CySC self-renewal. Finally, Hh signaling does not contribute to the niche function of CySCs, as Hh-sustained CySCs are unable to maintain GSCs in the absence of Stat92E. Therefore, the extended niche function of CySCs is solely attributable to JAK/STAT pathway function.
Figures

References
-
- Alcedo J., Ayzenzon M., Von Ohlen T., Noll M., Hooper J. E. (1996). The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86, 221–232 - PubMed
-
- Arbouzova N. I., Zeidler M. P. (2006). JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133, 2605–2616 - PubMed
-
- Aza-Blanc P., Ramírez-Weber F. A., Laget M. P., Schwartz C., Kornberg T. B. (1997). Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89, 1043–1053 - PubMed
-
- Azpiazu N., Lawrence P. A., Vincent J. P., Frasch M. (1996). Segmentation and specification of the Drosophila mesoderm. Genes Dev. 10, 3183–3194 - PubMed
-
- Bitgood M. J., Shen L., McMahon A. P. (1996). Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 6, 298–304 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

