Synchronous evolution of an odor biosynthesis pathway and behavioral response
- PMID: 23177478
- PMCID: PMC3543494
- DOI: 10.1016/j.cub.2012.10.047
Synchronous evolution of an odor biosynthesis pathway and behavioral response
Abstract
Background: Rodents use olfactory cues for species-specific behaviors. For example, mice emit odors to attract mates of the same species, but not competitors of closely related species. This implies rapid evolution of olfactory signaling, although odors and chemosensory receptors involved are unknown.
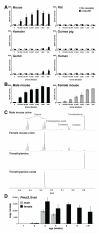
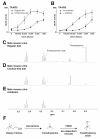
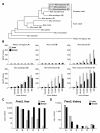
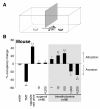
Results: Here, we identify a mouse chemosignal, trimethylamine, and its olfactory receptor, trace amine-associated receptor 5 (TAAR5), to be involved in species-specific social communication. Abundant (>1,000-fold increased) and sex-dependent trimethylamine production arose de novo along the Mus lineage after divergence from Mus caroli. The two-step trimethylamine biosynthesis pathway involves synergy between commensal microflora and a sex-dependent liver enzyme, flavin-containing monooxygenase 3 (FMO3), which oxidizes trimethylamine. One key evolutionary alteration in this pathway is the recent acquisition in Mus of male-specific Fmo3 gene repression. Coincident with its evolving biosynthesis, trimethylamine evokes species-specific behaviors, attracting mice, but repelling rats. Attraction to trimethylamine is abolished in TAAR5 knockout mice, and furthermore, attraction to mouse scent is impaired by enzymatic depletion of trimethylamine or TAAR5 knockout.
Conclusions: TAAR5 is an evolutionarily conserved olfactory receptor required for a species-specific behavior. Synchronized changes in odor biosynthesis pathways and odor-evoked behaviors could ensure species-appropriate social interactions.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
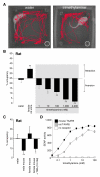
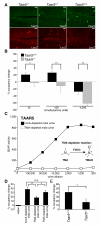
Comment in
-
Olfactory evolution: mice rethink stink.Curr Biol. 2013 Jan 21;23(2):R59-61. doi: 10.1016/j.cub.2012.11.051. Curr Biol. 2013. PMID: 23347937
Similar articles
-
Influence of Cat Odor on Reproductive Behavior and Physiology in the House Mouse: (Mus Musculus).In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 14. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 14. PMID: 24830030 Free Books & Documents. Review.
-
Sequence Variants in TAAR5 and Other Loci Affect Human Odor Perception and Naming.Curr Biol. 2020 Dec 7;30(23):4643-4653.e3. doi: 10.1016/j.cub.2020.09.012. Epub 2020 Oct 8. Curr Biol. 2020. PMID: 33035477
-
Deorphanization of Olfactory Trace Amine-Associated Receptors.Methods Mol Biol. 2025;2915:201-212. doi: 10.1007/978-1-0716-4466-9_14. Methods Mol Biol. 2025. PMID: 40249494
-
Combinatorial effects of odorants on mouse behavior.Proc Natl Acad Sci U S A. 2016 Jun 7;113(23):E3300-6. doi: 10.1073/pnas.1605973113. Epub 2016 May 20. Proc Natl Acad Sci U S A. 2016. PMID: 27208093 Free PMC article.
-
Odor-guided behavior in mammals.Experientia. 1986 Mar 15;42(3):257-71. doi: 10.1007/BF01942506. Experientia. 1986. PMID: 3514263 Review.
Cited by
-
Single olfactory receptors set odor detection thresholds.Nat Commun. 2018 Jul 23;9(1):2887. doi: 10.1038/s41467-018-05129-0. Nat Commun. 2018. PMID: 30038239 Free PMC article.
-
Sexual rejection via a vomeronasal receptor-triggered limbic circuit.Nat Commun. 2018 Oct 26;9(1):4463. doi: 10.1038/s41467-018-07003-5. Nat Commun. 2018. PMID: 30367054 Free PMC article.
-
Pigeon odor varies with experimental exposure to trace metal pollution.Ecotoxicology. 2019 Jan;28(1):76-85. doi: 10.1007/s10646-018-2001-x. Epub 2018 Nov 30. Ecotoxicology. 2019. PMID: 30506322
-
Coordination of two enhancers drives expression of olfactory trace amine-associated receptors.Nat Commun. 2021 Jun 18;12(1):3798. doi: 10.1038/s41467-021-23823-4. Nat Commun. 2021. PMID: 34145235 Free PMC article.
-
Neural and Hormonal Basis of Opposite-Sex Preference by Chemosensory Signals.Int J Mol Sci. 2021 Aug 2;22(15):8311. doi: 10.3390/ijms22158311. Int J Mol Sci. 2021. PMID: 34361077 Free PMC article. Review.
References
-
- Smadja C, Butlin RK. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity (Edinb) 2009;102:77–97. - PubMed
-
- Lassance JM, Groot AT, Lienard MA, Antony B, Borgwardt C, Andersson F, Hedenstrom E, Heckel DG, Lofstedt C. Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature. 2010;466:486–489. - PubMed
-
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. - PubMed
-
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature neuroscience. 2005;8:1660–1662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

