Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial
- PMID: 23177515
- PMCID: PMC3819942
- DOI: 10.1016/S0140-6736(12)61857-1
Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial
Abstract
Background: Until now, only imatinib and sunitinib have proven clinical benefit in patients with gastrointestinal stromal tumours (GIST), but almost all metastatic GIST eventually develop resistance to these agents, resulting in fatal disease progression. We aimed to assess efficacy and safety of regorafenib in patients with metastatic or unresectable GIST progressing after failure of at least imatinib and sunitinib.
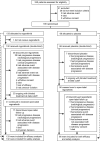
Methods: We did this phase 3 trial at 57 hospitals in 17 countries. Patients with histologically confirmed, metastatic or unresectable GIST, with failure of at least previous imatinib and sunitinib were randomised in a 2:1 ratio (by computer-generated randomisation list and interactive voice response system; preallocated block design (block size 12); stratified by treatment line and geographical region) to receive either oral regorafenib 160 mg daily or placebo, plus best supportive care in both groups, for the first 3 weeks of each 4 week cycle. The study sponsor, participants, and investigators were masked to treatment assignment. The primary endpoint was progression-free survival (PFS). At disease progression, patients assigned placebo could crossover to open-label regorafenib. Analyses were by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT01271712.
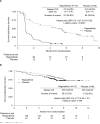
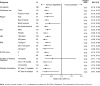
Results: From Jan 4, to Aug 18, 2011, 240 patients were screened and 199 were randomised to receive regorafenib (n=133) or matching placebo (n=66). Data cutoff was Jan 26, 2012. Median PFS per independent blinded central review was 4·8 months (IQR 1·4-9·2) for regorafenib and 0·9 months (0·9-1·8) for placebo (hazard ratio [HR] 0·27, 95% CI 0·19-0·39; p<0·0001). After progression, 56 patients (85%) assigned placebo crossed over to regorafenib. Drug-related adverse events were reported in 130 (98%) patients assigned regorafenib and 45 (68%) patients assigned placebo. The most common regorafenib-related adverse events of grade 3 or higher were hypertension (31 of 132, 23%), hand-foot skin reaction (26 of 132, 20%), and diarrhoea (seven of 132, 5%).
Interpretation: The results of this study show that oral regorafenib can provide a significant improvement in progression-free survival compared with placebo in patients with metastatic GIST after progression on standard treatments. As far as we are aware, this is the first clinical trial to show benefit from a kinase inhibitor in this highly refractory population of patients.
Funding: Bayer HealthCare Pharmaceuticals.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Evaluation of regorafenib in colorectal cancer and GIST.Lancet. 2013 Jan 26;381(9863):273-5. doi: 10.1016/S0140-6736(12)62006-6. Epub 2012 Nov 22. Lancet. 2013. PMID: 23177516 No abstract available.
-
Regorafenib, the CORRECT way forward or just another GRIDlock?Nat Rev Clin Oncol. 2013 Jan;10(1):1. doi: 10.1038/nrclinonc.2012.222. Epub 2012 Dec 11. Nat Rev Clin Oncol. 2013. PMID: 23229184 No abstract available.
References
-
- Joensuu H. Adjuvant treatment of GIST: patient selection and treatment strategies. Nat Rev Clin Oncol. 2012;9:351–58. - PubMed
-
- Edmonson JH, Marks RS, Buckner JC, Mahoney MR. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest. 2002;20:605–12. - PubMed
-
- Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–77. - PubMed
-
- Caram MV, Schuetze SM. Advanced or metastatic gastrointestinal stromal tumors: systemic treatment options. J Surg Oncol. 2011;104:888–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

