Higher iron in the red nucleus marks Parkinson's dyskinesia
- PMID: 23177595
- PMCID: PMC3570638
- DOI: 10.1016/j.neurobiolaging.2012.10.025
Higher iron in the red nucleus marks Parkinson's dyskinesia
Abstract
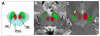
Dopamine cell loss and increased iron in the substantia nigra (SN) characterize Parkinson's disease (PD), with cerebellar involvement increasingly recognized, particularly in motor compensation and levodopa-induced dyskinesia (LID) development. Because the red nucleus (RN) mediates cerebellar circuitry, we hypothesized that RN iron changes might reflect cerebellum-related compensation, and/or the intrinsic capacity for LID development. We acquired high resolution magnetic resonance images from 23 control and 38 PD subjects (12 with PD and history of LID [PD+DYS]) and 26 with PD and no history of LID (PD-DYS). Iron content was estimated from bilateral RN and SN transverse relaxation rates (R2*). PD subjects overall displayed higher R2* values in both the SN and RN. RN R2* values correlated with off-drug Unified Parkinson's Disease Rating Scale-motor scores, but not disease duration or drug dosage. RN R2* values were significantly higher in PD+DYS compared with control and PD-DYS subjects; control and PD-DYS subjects did not differ. The association of higher RN iron content with PD-related dyskinesia suggests increased iron content is involved in, or reflects, greater cerebellar compensatory capacity and thus increased likelihood of LID development.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D, ILSA Working Group. Italian Longitudinal Study on Aging Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. Neurology. 2000;55:1358–1363. - PubMed
-
- Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr.Opin.Neurobiol. 2006;16:645–649. - PubMed
-
- Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–1472S. - PubMed
-
- Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr.Rev. 1993;51:157–170. - PubMed
-
- Belhaj-Saif A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol. 2000;83:3147–3153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

