Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis
- PMID: 23177622
- PMCID: PMC3606931
- DOI: 10.1016/j.celrep.2012.10.016
Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis
Abstract
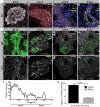
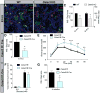
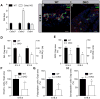
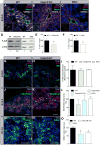
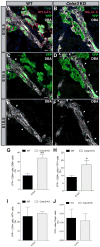
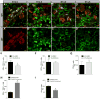
Planar cell polarity (PCP) refers to the collective orientation of cells within the epithelial plane. We show that progenitor cells forming the ducts of the embryonic pancreas express PCP proteins and exhibit an active PCP pathway. Planar polarity proteins are acquired at embryonic day 11.5 synchronously to apicobasal polarization of pancreas progenitors. Loss of function of the two PCP core components Celsr2 and Celsr3 shows that they control the differentiation of endocrine cells from polarized progenitors, with a prevalent effect on insulin-producing beta cells. This results in a decreased glucose clearance. Loss of Celsr2 and 3 leads to a reduction of Jun phosphorylation in progenitors, which, in turn, reduces beta cell differentiation from endocrine progenitors. These results highlight the importance of the PCP pathway in cell differentiation in vertebrates. In addition, they reveal that tridimensional organization and collective communication of cells are needed in the pancreatic epithelium in order to generate appropriate numbers of endocrine cells.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1 -deficient mice. Development. 1996;122:1409–1416. - PubMed
-
- Bacallao RL, McNeill H. Cystic kidney diseases and planar cell polarity signaling. Clin Genet. 2009;75:107–117. - PubMed
-
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. - PubMed
-
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

