Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors
- PMID: 23177962
- PMCID: PMC3509417
- DOI: 10.1016/j.neuron.2012.09.036
Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors
Abstract
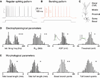
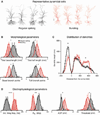
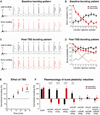
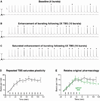
Relating the function of neuronal cell types to information processing and behavior is a central goal of neuroscience. In the hippocampus, pyramidal cells in CA1 and the subiculum process sensory and motor cues to form a cognitive map encoding spatial, contextual, and emotional information, which they transmit throughout the brain. Do these cells constitute a single class or are there multiple cell types with specialized functions? Using unbiased cluster analysis, we show that there are two morphologically and electrophysiologically distinct principal cell types that carry hippocampal output. We show further that these two cell types are inversely modulated by the synergistic action of glutamate and acetylcholine acting on metabotropic receptors that are central to hippocampal function. Combined with prior connectivity studies, our results support a model of hippocampal processing in which the two pyramidal cell types are predominantly segregated into two parallel pathways that process distinct modalities of information.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Babadi B. Bursting as an effective relay mode in a minimal thalamic model. J Comput Neurosci. 2005;18:229–243. - PubMed
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

