Synchronous oscillatory neural ensembles for rules in the prefrontal cortex
- PMID: 23177967
- PMCID: PMC3907768
- DOI: 10.1016/j.neuron.2012.09.029
Synchronous oscillatory neural ensembles for rules in the prefrontal cortex
Abstract
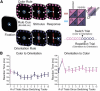
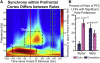
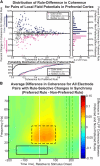
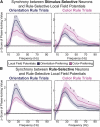
Intelligent behavior requires acquiring and following rules. Rules define how our behavior should fit different situations. To understand its neural mechanisms, we simultaneously recorded from multiple electrodes in dorsolateral prefrontal cortex (PFC) while monkeys switched between two rules (respond to color versus orientation). We found evidence that oscillatory synchronization of local field potentials (LFPs) formed neural ensembles representing the rules: there were rule-specific increases in synchrony at "beta" (19-40 Hz) frequencies between electrodes. In addition, individual PFC neurons synchronized to the LFP ensemble corresponding to the current rule (color versus orientation). Furthermore, the ensemble encoding the behaviorally dominant orientation rule showed increased "alpha" (6-16 Hz) synchrony when preparing to apply the alternative (weaker) color rule. This suggests that beta-frequency synchrony selects the relevant rule ensemble, while alpha-frequency synchrony deselects a stronger, but currently irrelevant, ensemble. Synchrony may act to dynamically shape task-relevant neural ensembles out of larger, overlapping circuits.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Prefrontal α- and β-band oscillations are involved in rule selection.Trends Cogn Sci. 2013 Jan;17(1):10-2. doi: 10.1016/j.tics.2012.11.002. Epub 2012 Nov 21. Trends Cogn Sci. 2013. PMID: 23176827
-
Rules got rhythm.Neuron. 2012 Nov 21;76(4):673-6. doi: 10.1016/j.neuron.2012.11.003. Neuron. 2012. PMID: 23177954
-
Cognitive neuroscience: Rules of neural engagement.Nat Rev Neurosci. 2013 Jan;14(1):1. doi: 10.1038/nrn3417. Epub 2012 Dec 12. Nat Rev Neurosci. 2013. PMID: 23232605 No abstract available.
References
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J. Neurophysiol. 1989;61:900–917. - PubMed
-
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: Exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and Performance XV: Conscious and Nonconscious Information Processing. The MIT Press; Cambridge, MA: 1994. pp. 421–452.
-
- Androulidakis AG, Doyle LMF, Yarrow K, Litvak V, Gilbertson TP, Brown P. Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. Eur. J. Neurosci. 2007;25:3758–3765. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
