Effects of the activin A-myostatin-follistatin system on aging bone and muscle progenitor cells
- PMID: 23178301
- PMCID: PMC3678732
- DOI: 10.1016/j.exger.2012.11.004
Effects of the activin A-myostatin-follistatin system on aging bone and muscle progenitor cells
Abstract
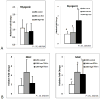
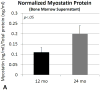
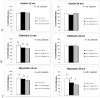
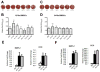
The activin A-myostatin-follistatin system is thought to play an important role in the regulation of muscle and bone mass throughout growth, development, and aging; however, the effects of these ligands on progenitor cell proliferation and differentiation in muscle and bone are not well understood. In addition, age-associated changes in the relative expression of these factors in musculoskeletal tissues have not been described. We therefore examined changes in protein levels of activin A, follistatin, and myostatin (GDF-8) in both muscle and bone with age in C57BL6 mice using ELISA. We then investigated the effects of activin A, myostatin and follistatin on the proliferation and differentiation of primary myoblasts and mouse bone marrow stromal cells (BMSCs) in vitro. Myostatin levels and the myostatin:follistatin ratio increased with age in the primarily slow-twitch mouse soleus muscle, whereas the pattern was reversed with age in the fast-twitch extensor digitorum longus muscle. Myostatin levels and the myostatin:follistatin ratio increased significantly (+75%) in mouse bone marrow with age, as did activin A levels (+17%). Follistatin increased the proliferation of primary myoblasts from both young and aged mice, whereas myostatin increased proliferation of younger myoblasts but decreased proliferation of older myoblasts. Myostatin reduced proliferation of both young and aged BMSCs in a dose-dependent fashion, and activin A increased mineralization in both young and aged BMSCs. Together these data suggest that aging in mice is accompanied by changes in the expression of activin A and myostatin, as well as changes in the response of bone and muscle progenitor cells to these factors. Myostatin appears to play a particularly important role in the impaired proliferative capacity of muscle and bone progenitor cells from aged mice.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Baccarelli A, Morpurgo P, Corsi A, Vaghi I, Fanelli M, Cremonesi G, Vaninetti S, Beck-Peccoz P, Spada A. Activin A serum levels and aging of the pituitary-gonadal axis: a cross-sectional study in middle-aged and elderly healthy subjects. Exp Gerontol. 2001;36:1403–12. - PubMed
-
- Conboy I, Conboy MJ, Smythe G, Rando TM. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–177. - PubMed
-
- Dalbo VJ, Roberts MD, Sunderland KL, Poole CN, Stout JR, Beck TW, Bemben M, Kerksick CM. Acute loading and aging effects on myostatin pathway biomarkers in human skeletal muscle after three sequential bouts of resistance exercise. J Gerontol A Biol Sci Med Sci. 2011;66:855–65. - PubMed
-
- Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, van der Spek PJ, Visser JA, de Jong FH, Pols HA, van Leeuwen JP. The activin A-follistatin system: potent regulator of human extracellular matrix mineralization. FASEB J. 2007;21:2949–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

