Site-specific monoubiquitination activates Ras by impeding GTPase-activating protein function
- PMID: 23178454
- PMCID: PMC3537887
- DOI: 10.1038/nsmb.2430
Site-specific monoubiquitination activates Ras by impeding GTPase-activating protein function
Abstract
Cell growth and differentiation are controlled by growth factor receptors coupled to the GTPase Ras. Oncogenic mutations disrupt GTPase activity, leading to persistent Ras signaling and cancer progression. Recent evidence indicates that monoubiquitination of Ras leads to Ras activation. Mutation of the primary site of monoubiquitination impairs the ability of activated K-Ras (one of the three mammalian isoforms of Ras) to promote tumor growth. To determine the mechanism of human Ras activation, we chemically ubiquitinated the protein and analyzed its function by NMR, computational modeling and biochemical activity measurements. We established that monoubiquitination has little effect on the binding of Ras to guanine nucleotide, GTP hydrolysis or exchange-factor activation but severely abrogates the response to GTPase-activating proteins in a site-specific manner. These findings reveal a new mechanism by which Ras can trigger persistent signaling in the absence of receptor activation or an oncogenic mutation.
Figures

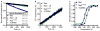
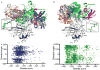



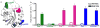
Comment in
-
Cell signalling: How monoubiquitylation keeps RAS active.Nat Rev Mol Cell Biol. 2013 Feb;14(2):66-7. doi: 10.1038/nrm3500. Epub 2012 Dec 19. Nat Rev Mol Cell Biol. 2013. PMID: 23249902 No abstract available.
References
-
- Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. - PubMed
-
- Kjeldgaard M, Nyborg J, Clark BF. The GTP binding motif: variations on a theme. FASEB J. 1996;10:1347–68. - PubMed
-
- Herrmann C. Ras–effector interactions: after one decade. Curr Opin Struct Biol. 2003;13:122–129. - PubMed
-
- Scheffzek K, et al. The Ras–RasGAP Complex: Structural Basis for GTPase Activation and Its Loss in Oncogenic Ras Mutants. Science. 1997;277:333–339. - PubMed
-
- Sprang S. GEFs: master regulators of G–protein activation. Trends Biochem Sci. 2001;26:266–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM106227/GM/NIGMS NIH HHS/United States
- R01GM073180-06S1/GM/NIGMS NIH HHS/United States
- T32 GM008570/GM/NIGMS NIH HHS/United States
- R01 GM073151/GM/NIGMS NIH HHS/United States
- R01CA089614/CA/NCI NIH HHS/United States
- R01GM073151/GM/NIGMS NIH HHS/United States
- R01GM41890/GM/NIGMS NIH HHS/United States
- R01GM073960/GM/NIGMS NIH HHS/United States
- R01 CA089614/CA/NCI NIH HHS/United States
- T32GM008570/GM/NIGMS NIH HHS/United States
- R01 GM073180/GM/NIGMS NIH HHS/United States
- R01 GM073960/GM/NIGMS NIH HHS/United States
- R01 GM056203/GM/NIGMS NIH HHS/United States
- P01 CA117969/CA/NCI NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- P01CA117969/CA/NCI NIH HHS/United States
- R01 GM041890/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

