Development of a Coxsackievirus A16 neutralization assay based on pseudoviruses for measurement of neutralizing antibody titer in human serum
- PMID: 23178532
- PMCID: PMC7112850
- DOI: 10.1016/j.jviromet.2012.11.014
Development of a Coxsackievirus A16 neutralization assay based on pseudoviruses for measurement of neutralizing antibody titer in human serum
Abstract
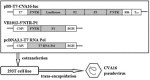
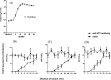
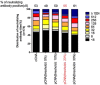
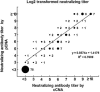
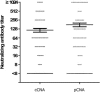
Serum neutralizing antibody titers are indicative of protective immunity against Coxsackievirus A16 (CV-A16) and Enterovirus 71 (EV71), the two main etiological agents of hand, foot and mouth disease (HFMD), and provide the basis for evaluating vaccine efficacy. The current CV-A16 neutralization assay based on inhibition of cytopathic effects requires manual microscopic examination, which is time-consuming and labor-intensive. In this study, a high-throughput neutralization assay was developed by employing CV-A16 pseudoviruses expressing luciferase for detecting infectivity in rhabdomyosarcoma (RD) cells and measuring serum viral neutralizing antibodies. Without the need to use infectious CV-A16 strains, the neutralizing antibody titer against CV-A16 could be determined within 15h by measuring luciferase signals by this assay. The pseudovirus CV-A16 neutralization assay (pCNA) was validated by comparison with a conventional CV-A16 neutralization assay (cCNA) in testing 174 human serum samples collected from children (age <5 years). The neutralizing antibody titers determined by these two assays were well correlated (R(2)=0.7689). These results suggest that the pCNA can serve as a rapid and objective procedure for the measurement of neutralizing antibodies against CV-A16.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

References
-
- Ang L.W., Koh B.K., Chan K.P., Chua L.T., James L., Goh K.T. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001–2007. Ann. Acad. Med. Singapore. 2009;38:106–112. - PubMed
-
- Bartosch B., Bukh J., Meunier J.C., Granier C., Engle R.E., Blackwelder W.C., Emerson S.U., Cosset F.L., Purcell R.H. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14199–14204. - PMC - PubMed
-
- Chang L.Y., King C.C., Hsu K.H., Ning H.C., Tsao K.C., Li C.C., Huang Y.C., Shih S.R., Chiou S.T., Chen P.Y., Chang H.J., Lin T.Y. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109:e88. - PubMed
-
- Chang L.Y., Lin T.Y., Huang Y.C., Tsao K.C., Shih S.R., Kuo M.L., Ning H.C., Chung P.W., Kang C.M. Comparison of enterovirus 71 and coxsackie-virus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr. Infect. Dis. J. 1999;18:1092–1096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

