Choice-related activity and correlated noise in subcortical vestibular neurons
- PMID: 23178975
- PMCID: PMC3612962
- DOI: 10.1038/nn.3267
Choice-related activity and correlated noise in subcortical vestibular neurons
Abstract
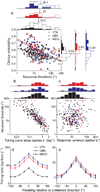
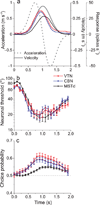
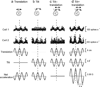
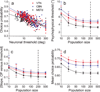
Functional links between neuronal activity and perception are studied by examining trial-by-trial correlations (choice probabilities) between neural responses and perceptual decisions. We addressed fundamental issues regarding the nature and origin of choice probabilities by recording from subcortical (brainstem and cerebellar) neurons in rhesus monkeys during a vestibular heading discrimination task. Subcortical neurons showed robust choice probabilities that exceeded those seen in cortex (area MSTd) under identical conditions. The greater choice probabilities of subcortical neurons could be predicted by a stronger dependence of correlated noise on tuning similarity, as revealed by population decoding. Significant choice probabilities were observed almost exclusively for neurons that responded selectively to translation, whereas neurons that represented net gravito-inertial acceleration did not show choice probabilities. These findings suggest that the emergence of choice probabilities in the vestibular system depends on a critical signal transformation that occurs in subcortical pathways to distinguish translation from orientation relative to gravity.
Figures




References
-
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. - PubMed
-
- Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci. 2005;8:99–106. - PubMed
-
- Uka T, DeAngelis GC. Contribution of area MT to stereoscopic depth perception: choice-related response modulations reflect task strategy. Neuron. 2004;42:297–310. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

