Enzyme-catalyzed protein crosslinking
- PMID: 23179622
- PMCID: PMC3546294
- DOI: 10.1007/s00253-012-4569-z
Enzyme-catalyzed protein crosslinking
Abstract
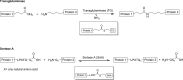
The process of protein crosslinking comprises the chemical, enzymatic, or chemoenzymatic formation of new covalent bonds between polypeptides. This allows (1) the site-directed coupling of proteins with distinct properties and (2) the de novo assembly of polymeric protein networks. Transferases, hydrolases, and oxidoreductases can be employed as catalysts for the synthesis of crosslinked proteins, thereby complementing chemical crosslinking strategies. Here, we review enzymatic approaches that are used for protein crosslinking at the industrial level or have shown promising potential in investigations on the lab-scale. We illustrate the underlying mechanisms of crosslink formation and point out the roles of the enzymes in their natural environments. Additionally, we discuss advantages and drawbacks of the enzyme-based crosslinking strategies and their potential for different applications.
Figures



References
-
- Ando H, Adachi M, Umeda K, Matsuura A, Nonaka M, Uchio R, Tanaka H, Motoki M. Purification and characteristics of a novel transglutaminase derived from microorganisms. Agr Biol Chem Tokyo. 1989;53:2613–2617. doi: 10.1271/bbb1961.53.2613. - DOI
-
- Basman A, Köksel H, Ng PKW. Effects of transglutaminase on SDS-PAGE patterns of wheat, soy, and barley proteins and their blends. J Food Sci. 2002;67:2654–2658. doi: 10.1111/j.1365-2621.2002.tb08794.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

