Imaging characteristics of cerebrovascular arteriopathy and stroke in Hutchinson-Gilford progeria syndrome
- PMID: 23179651
- PMCID: PMC7964639
- DOI: 10.3174/ajnr.A3341
Imaging characteristics of cerebrovascular arteriopathy and stroke in Hutchinson-Gilford progeria syndrome
Abstract
Background and purpose: HGPS is a rare disorder of segmental aging, with early morbidity from cardiovascular and cerebrovascular disease. The goal of this study was to identify the neurovascular features, infarct type, topography, and natural history of stroke in the only neurovascular imaging cohort study of HGPS.
Materials and methods: We studied 25 children with confirmed diagnoses of HGPS and neuroimaging studies available for review. Relevant clinical information was abstracted from medical records.
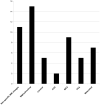
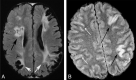
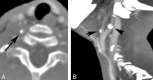
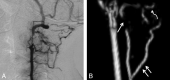
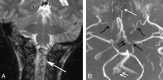
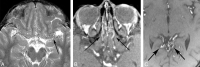
Results: We identified features suggestive of a vasculopathy unique to HGPS, including distinctive intracranial steno-occlusive arterial lesions, basal cistern collateral vessels, and slow compensatory collateral flow over the cerebral convexities. The arterial pathology in the neck consisted of distal vertebral artery stenosis with prominent collateral vessel formation as well as stenosis and calcification of both the cervical internal and common carotid arteries. Radiographic evidence of infarction was found in 60% of patients, of which half were likely clinically silent. Both large- and small-vessel disease was observed, characterized by arterial territorial, white matter, lacunar, and watershed infarcts.
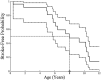
Conclusions: We report a unique intracranial and superior cervical arteriopathy in HGPS distinct from other vasculopathies of childhood, such as Moyamoya, and cerebrovascular disease of aging, including atherosclerosis. Arterial features of the mid and lower neck are less distinctive. For the first time, we identified early and clinically silent strokes as a prevalent disease characteristic in HGPS. Longitudinal analysis of stroke incidence and vasculopathy may provide an outcome measure for future treatment interventions for children with HGPS.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
