Applications of minimal physiologically-based pharmacokinetic models
- PMID: 23179857
- PMCID: PMC3539784
- DOI: 10.1007/s10928-012-9280-2
Applications of minimal physiologically-based pharmacokinetic models
Abstract
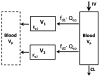
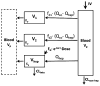
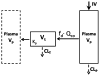
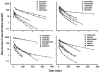
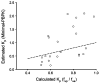
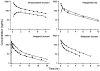
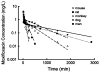
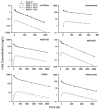
Conventional mammillary models are frequently used for pharmacokinetic (PK) analysis when only blood or plasma data are available. Such models depend on the quality of the drug disposition data and have vague biological features. An alternative minimal-physiologically-based PK (minimal-PBPK) modeling approach is proposed which inherits and lumps major physiologic attributes from whole-body PBPK models. The body and model are represented as actual blood and tissue (usually total body weight) volumes, fractions (f ( d )) of cardiac output with Fick's Law of Perfusion, tissue/blood partitioning (K ( p )), and systemic or intrinsic clearance. Analyzing only blood or plasma concentrations versus time, the minimal-PBPK models parsimoniously generate physiologically-relevant PK parameters which are more easily interpreted than those from mammillary models. The minimal-PBPK models were applied to four types of therapeutic agents and conditions. The models well captured the human PK profiles of 22 selected beta-lactam antibiotics allowing comparison of fitted and calculated K ( p ) values. Adding a classical hepatic compartment with hepatic blood flow allowed joint fitting of oral and intravenous (IV) data for four hepatic elimination drugs (dihydrocodeine, verapamil, repaglinide, midazolam) providing separate estimates of hepatic intrinsic clearance, non-hepatic clearance, and pre-hepatic bioavailability. The basic model was integrated with allometric scaling principles to simultaneously describe moxifloxacin PK in five species with common K ( p ) and f ( d ) values. A basic model assigning clearance to the tissue compartment well characterized plasma concentrations of six monoclonal antibodies in human subjects, providing good concordance of predictions with expected tissue kinetics. The proposed minimal-PBPK modeling approach offers an alternative and more rational basis for assessing PK than compartmental models.
Figures
References
-
- Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. - PubMed
-
- Jusko WJ. Guidelines for collection and pharmacokinetic analysis of drug disposition data. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. 1. Applied Therapeutics Inc; Vancouver, WA: 1980. pp. 639–680.
-
- Chiou WL. Potential pitfalls in the conventional pharmacokinetic studies: effects of the initial mixing of drug in blood and the pulmonary first-pass elimination. J Pharmacokinet Biopharm. 1979;7:527–536. - PubMed
-
- Riegelman S, Loo JC, Rowland M. Shortcomings in pharmacokinetic analysis by conceiving the body to exhibit properties of a single compartment. J Pharm Sci. 1968;57:117–123. - PubMed
-
- Vaughan DP, Hope I. Applications of a recirculatory stochastic pharmacokinetic model: limitations of compartmental models. J Pharmacokinet Biopharm. 1979;7:207–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

