Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-κB impairs this drug-induced senescence
- PMID: 23180582
- PMCID: PMC3569660
- DOI: 10.1002/emmm.201201378
Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-κB impairs this drug-induced senescence
Abstract
Oncogene-induced senescence can provide a protective mechanism against tumour progression. However, production of cytokines and growth factors by senescent cells may contribute to tumour development. Thus, it is unclear whether induction of senescence represents a viable therapeutic approach. Here, using a mouse model with orthotopic implantation of metastatic melanoma tumours taken from 19 patients, we observed that targeting aurora kinases with MLN8054/MLN8237 impaired mitosis, induced senescence and markedly blocked proliferation in patient tumour implants. Importantly, when a subset of tumour-bearing mice were monitored for tumour progression after pausing MLN8054 treatment, 50% of the tumours did not progress over a 12-month period. Mechanistic analyses revealed that inhibition of aurora kinases induced polyploidy and the ATM/Chk2 DNA damage response, which mediated senescence and a NF-κB-related, senescence-associated secretory phenotype (SASP). Blockade of IKKβ/NF-κB led to reversal of MLN8237-induced senescence and SASP. Results demonstrate that removal of senescent tumour cells by infiltrating myeloid cells is crucial for inhibition of tumour re-growth. Altogether, these data demonstrate that induction of senescence, coupled with immune surveillance, can limit melanoma growth.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures

A,B. Biopsy tumour tissues from 19 melanoma patients were implanted into nude mice and when tumours formed they were passaged by implantation into treatment groups (n ≥ 4 mice per group). Tumour-bearing mice received MLN8054 (60 mg/kg) or vehicle alone (A), MLN8237 (30 mg/kg) or vehicle alone (B), once daily by oral gavage for 2–6 weeks depending on their response to the treatment or the tumour volume. Mean tumour volumes ± SEM are shown for patient V13 (A) or V35 (B) as representative patient tumours.
C. Hs294T melanoma cells were injected subcutaneously into nude mice (2 × 106 cells per mouse). After 1 week, tumour-bearing mice were treated with vehicle control or MLN8237 (30 mg/kg) once daily for 28 days. Tumour volume was then evaluated to determine response to the drug. Mean tumour volumes ± SEM for five mice per treatment group are shown.
D. Tissue microarray (TMA) slides from patient melanoma tumour implants growing on mice treated with vehicle control or MLN8237 were stained for pAURKA (T-288) (red), alpha tubulin (green) and DAPI (blue) to identify cells with p-AURKA associated with the nuclear spindle microtubule complex (yellow) (see insert enlargement in upper panel).
E. Histological features of the H&E stained patient melanoma tissues from mice treated with vehicle or MLN8237.
F. Ki67 (proliferation marker) staining of patient tissues from mice treated with MLN8237 or vehicle control.

Hs294T (p53WT), SK-Mel-2 (p53 mutant), SK-Mel-5 (p53WT) and SK-Mel-28 (p53 mutant) cells were treated with 1 µM MLN8237 for 6 or 12 days. After treatment, viable cells were counted and compared to the initial cell number. Data indicate mean values ± SD (n = 3) from one representative of three independent experiments. p-value shown represents difference between treated group and day 0 control group (Student's t-test).
Cultures of Hs294T, SK-Mel-2, SK-Mel-5 and SK-Mel-28 cells were treated with MLN8237 or vehicle for indicated time points and apoptosis was analysed by FACS analysis for propidium iodide (PI) and Annexin V staining. Data indicate mean values ± SD (n = 3) from triplicate experiments.
Hs294T cells were treated with 1 µM MLN8237 or vehicle for 5 days and the change in morphology was captured with an AxioVision microscope.
Hs294T cells were treated with 1 µM MLN8237 for 5 days, and senescence was determined by β-galactosidase staining.
Melanoma cell lines with wild-type p53 (Hs294T and SK-Mel-5) or mutated p53 (SK-Mel-2 and SK-Mel-28) were treated with 1 µM or 5 µM MLN8237 for 5 days. After treatment, p53, p63, p73, p21, p16 and GAPDH were analysed by Western blot.
Hs294T and SK-Mel-28 cells were treated with 1 µM MLN8237 or vehicle in the presence or absence of the p53 inhibitor pifithrin-α (PTF-α, 5 µM) or DMSO for 5 days. After treatment, β-galactosidase staining was performed. All experiments were conducted at least three times independently with reproducible results.

A. Hs294T cells were treated with 1 µM MLN8237 or vehicle control for 2 days and DNA content was examined by FACS.
B. Hs294T cells were treated with 1 µM MLN8237 or vehicle control for 5 days. After treatment, 53BP1 and γ-H2A.X were evaluated by immunocytochemistry. Cell nuclei were counterstained with Hoechst dye, and the samples were visualized by microscopy.
C. Hs294T, SK-Mel-2, SK-Mel-5 and SK-Mel-28 cells were treated with vehicle alone, 1 or 5 µM MLN8237 for 5 days, and the levels of γ-H2AX, p-Chk2, Chk2, p-Chk1 and Chk1 were analysed by Western blot.
D,E. Hs294T cells and SK-Mel-28 cells were treated with 1 µM MLN8237 with or without the ATM inhibitor KU-55933 at the indicated concentrations for 48 h. After treatment, the level of p-Chk2 was examined by Western blot (D) and senescence was determined by β-galactosidase staining (E).
F–H. Hs294T cells and SK-Mel-28 cells were transfected with siATM or siChk2. Twenty-four hours after transfection, cells were treated with 1 µM MLN8237 for 3 days. Levels of ATM (F), p-Chk2 and Chk2 (G) were analysed by Western blot. Senescence after transfection of siATM or siChk2 was evaluated by β-galactosidase staining and representative photomicrographs are shown (H). One hundred cells were counted from three independent experiments and mean % senescent cells is noted in each micrograph.

Hs294T cells were pre-treated with 1 µM MLN8237 or vehicle control for 5 days. After treatment, cytokine secretion into the medium was assayed by cytokine array.
Hs294T, SK-Mel-2, SK-Mel-5 and SK-Mel-28 cells were treated with 1 or 5 µM MLN8237 for 5 days and the levels of p-p65 (S536) and p65 were analysed by Western blot. Hs294T, SK-Mel-2, SK-Mel-5 and SK-Mel-28 cells were treated with 1 µM MLN8237 for 3 days or 5 days, and the level of IκB-α was analysed by Western blot.
NF-κB reporter-stable Hs294T cells were treated with 1 µM MLN8237 for 5 days. After treatment, NF-κB transcriptional activity was measured by luciferase assay and the results were normalized by cell number. Data indicate mean values ± SD (n = 3) from a representative experiment performed three times.
Hs294T cells were treated with 1 or 5 µM MLN8237 for 5 days. Levels of phospho-AKT (p-AKT), total AKT, phospho-ERK (p-ERK), total ERK, phospho-p38 MAPK (p-p38), total p38, phospho-STAT3 (p-STAT3), total STAT3 and GAPDH were measured by Western blot.
Conditioned medium from senescent Hs294T cells was added to the bottom wells in 96 transwell cell-plates. 200 µl (106 cells/ml) of dHL60 cells (human promyelocytic leukcmia cells differentiated along a neutrophil cell lineage) were seeded in the chemotaxis chamber. The chamber was incubated at 37°C 5% CO2 for 1 h, then the transmigrated cells were counted by haemocytometer. Data indicate mean values ± SD (n = 4).

A. The tissue level of senescence in an Hs294T xenograft treated with MLN8237 or vehicle control was determined by β-galactosidase staining.
B–D. Tissue levels of 53BP1, α-tubulin, IκB-α and IL-6 in an Hs294T xenograft treated with MLN8237 or vehicle control were visualized by immunofluorescence co-staining with DAPI. Representative micrographs are shown from triplicate experiments.
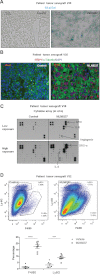
The tissue level of senescence in a melanoma patient implant (V35) after mice were treated with MLN8237 or vehicle was determined by β-galactosidase staining.
To assess DNA damage, 53BP1 (red) was visualized by immunofluorescence and tissues were co-stained with α-tubulin (green) and DAPI (blue). A and B were analysed from multiple slides and representative images are shown.
Cytokine profile of MLN8237-treated tumour tissue was analysed in tumour tissue lysates using cytokine array.
The infiltrating neutrophils and macrophages were evaluated by FACS using anti-Ly-6G and F4/80 antibodies, respectively. Seven tumours were analysed from each group. Means ± SEM are shown. p-value: ****9.16945E−06; ***0.000573.

The mouse melanoma line, MelA, was treated with MLN8237 for 5 days and senescence was determined by β-galactosidase staining.
MelA cells were pre-treated with MLN8237 for 1 week to induce senescence. Macrophages were depleted with 1 mg of clodronate in C57BL/6 mice. One day after clodronate treatment (macrophage depletion), 5 × 106 MLN8237-pre-treated MelA cells were injected into C57BL/6 mice. Control mice were treated with liposome vehicle (n = 5). Non-pre-treated MelA cells were also injected into C57BL/6 mice with or without macrophage depletion as controls (n = 7). Tumour formation was monitored in each group of mice for 17 days.
Tumour volume was measured once per week. The scatter plot shows the tumour volume for each mouse at day 8 and Day 17.

Hs294T cells were transfected with IKKβ siRNA, and knockdown of IKKβ verified by Western blot. siRNA control or siIKKβ transfected cells were treated with 1 µM MLN8237 for 5 days, and senescence was evaluated by β-galactosidase staining.
Hs294T and SK-Mel-28 cells were treated with the IKKβ inhibitor BMS-345541 (10 µM) for 48 h, and the level of p-p65 and p65 were analysed by Western blot.
Hs294T cells were treated with 1 µM MLN8237 with or without 10 µM BMS-34554 for 5 days. After treatment, viable cells were counted and 5 × 105 cells were seeded into 10-cm plates in DMEM F-12 with 10% FBS. Once cells attached, serum-containing medium was replaced with serum-free medium and cells were cultured overnight. Cytokine secretion into the medium was assayed by cytokine array.
Hs294T and SK-Mel-28 cells were treated with 10 µM BMS-345541, 1 µM MLN8237, or both for 2 days and DNA content was examined by FACS.
Hs294T and SK-Mel-28 cells were treated with 10 µM BMS-345541, 1 µM MLN8237, or both for 5 days. After treatment, senescence was evaluated by β-galactosidase staining.
Hs294T and SK-Mel-28 cells were treated with 10 µM BMS-345541, 1 µM MLN8237, or both for 5 days, and the viable cells were counted using a haemocytometer. Data indicate mean values ± SD (n = 3). With the exception of Fig 8C, all experiments were performed in triplicate with high reproducibility and representative experiments are shown.
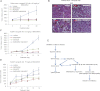
A. Patient tumour tissues (V32) were implanted subcutaneously into nude mice. After 1 week, tumour-bearing mice received daily oral doses of BMS-345541 (100 mg/kg) or MLN8237 (30 mg/kg) or both. Mean tumour volumes ± SEM are shown (n = 5).
B. H&E staining of the V32 patient tumours from mice treated with vehicle, BMS345541, MLN8237, or both BMS345541 and MLN8237.
C,D. Hs294T melanoma cells were injected subcutaneously into nude mice (2 × 106 cells per mouse). After 1 week, tumour-bearing mice received daily oral doses of BMS-345541 [100 mg/kg (C) or 75 mg/kg (D)] or MLN8237 (30 mg/kg) or 30 mg/kg MLN8237 combined with 100 mg/kg BMS345541 (C) or 75 mg/kg BMS345541 (D). Mean tumour volumes ± SEM are shown. (n = 5).
E. Diagrammatic representation of the proposed model of MLN8237-induced senescence and senescence surveillance by immune cells.
Similar articles
-
Senescence-associated secretory factors induced by cisplatin in melanoma cells promote non-senescent melanoma cell growth through activation of the ERK1/2-RSK1 pathway.Cell Death Dis. 2018 Feb 15;9(3):260. doi: 10.1038/s41419-018-0303-9. Cell Death Dis. 2018. PMID: 29449532 Free PMC article.
-
Combining an Aurora Kinase Inhibitor and a Death Receptor Ligand/Agonist Antibody Triggers Apoptosis in Melanoma Cells and Prevents Tumor Growth in Preclinical Mouse Models.Clin Cancer Res. 2015 Dec 1;21(23):5338-48. doi: 10.1158/1078-0432.CCR-15-0293. Epub 2015 Jul 7. Clin Cancer Res. 2015. PMID: 26152738 Free PMC article.
-
A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma.Blood. 2010 Jun 24;115(25):5202-13. doi: 10.1182/blood-2009-12-259523. Epub 2010 Apr 9. Blood. 2010. PMID: 20382844 Free PMC article.
-
Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer.Aging (Albany NY). 2011 Nov;3(11):1063-77. doi: 10.18632/aging.100407. Aging (Albany NY). 2011. PMID: 22170748 Free PMC article. Review.
-
How to activate p53.Curr Biol. 2000 Apr 20;10(8):R315-7. doi: 10.1016/s0960-9822(00)00439-5. Curr Biol. 2000. PMID: 10801407 Review.
Cited by
-
Cancer treatment-induced NAD+ depletion in premature senescence and late cardiovascular complications.J Cardiovasc Aging. 2022;2:28. doi: 10.20517/jca.2022.13. Epub 2022 Apr 29. J Cardiovasc Aging. 2022. PMID: 35801078 Free PMC article.
-
Cell-state dynamics and therapeutic resistance in melanoma from the perspective of MITF and IFNγ pathways.Nat Rev Clin Oncol. 2019 Sep;16(9):549-562. doi: 10.1038/s41571-019-0204-6. Nat Rev Clin Oncol. 2019. PMID: 30967646 Free PMC article. Review.
-
BID expression determines the apoptotic fate of cancer cells after abrogation of the spindle assembly checkpoint by AURKB or TTK inhibitors.Mol Cancer. 2023 Jul 13;22(1):110. doi: 10.1186/s12943-023-01815-w. Mol Cancer. 2023. PMID: 37443114 Free PMC article.
-
MDM2 Antagonists Counteract Drug-Induced DNA Damage.EBioMedicine. 2017 Oct;24:43-55. doi: 10.1016/j.ebiom.2017.09.016. Epub 2017 Sep 19. EBioMedicine. 2017. PMID: 29030058 Free PMC article.
-
Inhibition of Aurora Kinase B activity disrupts development and differentiation of salivary glands.Cell Death Discov. 2021 Jan 18;7(1):16. doi: 10.1038/s41420-020-00393-w. Cell Death Discov. 2021. PMID: 33462217 Free PMC article.
References
-
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Bennecke M, Kriegl L, Bajbouj M, Retzlaff K, Robine S, Jung A, Arkan MC, Kirchner T, Greten FR. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell. 2010;18:135–146. - PubMed
-
- Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–418. - PubMed
-
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- IK6 BX005225/BX/BLRD VA/United States
- K12-CA090625/CA/NCI NIH HHS/United States
- 2 UL1 TR000445-06/TR/NCATS NIH HHS/United States
- R01 FD003522/FD/FDA HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA116021/CA/NCI NIH HHS/United States
- K12 GM068543/GM/NIGMS NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- CA116021/CA/NCI NIH HHS/United States
- U54 CA163072/CA/NCI NIH HHS/United States
- T32 CA119925/CA/NCI NIH HHS/United States
- K12 CA090625/CA/NCI NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- 5P30CA068485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- T32 HL007751/HL/NHLBI NIH HHS/United States
- T32 119925/PHS HHS/United States
- T32HL07751/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

