NLRP3 inflammasome activation induced by engineered nanomaterials
- PMID: 23180683
- PMCID: PMC4056676
- DOI: 10.1002/smll.201201962
NLRP3 inflammasome activation induced by engineered nanomaterials
Abstract
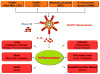
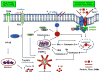
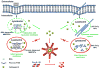
Engineered nanomaterials (ENMs) continue to attract significant attention because they have novel physicochemical properties that can improve the functions of products that will benefit human lives. However, the physicochemical properties that make ENMs attractive could interact with biological systems and induce cascades of events that cause toxicological effects. Recently, there have been more studies suggesting inflammasome activation may play an important role in ENM-induced biological responses. Inflammasomes are a family of multiprotein complexes that are increasingly recognized as major mediators of the host immune system. Among these, NLRP3 inflammasome is the most studied that could directly interact with ENMs to generate inflammatory responses. In this review, the ENM physicochemical properties are linked to NLRP3 inflammasome activation. An understanding of the mechanisms of ENM-NLRP3 inflammasome interactions will provide us with strategies for safer nanomaterial design and therapy.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

