Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network
- PMID: 23180825
- PMCID: PMC3574288
- DOI: 10.1096/fj.12-219618
Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network
Abstract
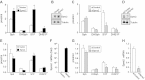
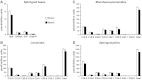
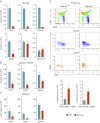
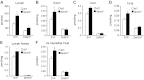
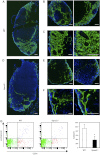
Sphingosine-1-phosphate (S1P), a ligand for 5 specific receptors, is a potent lipid mediator that plays important roles in lymphocyte trafficking and immune responses. S1P is produced inside cells and therefore must be secreted to exert its effects through these receptors. Spinster 2 (Spns2) is one of the cell surface transporters thought to secrete S1P. We have shown that Spns2 can export endogenous S1P from cells and also dihydro-S1P, which is active at all cell surface S1P receptors. Moreover, Spns2 mice have decreased levels of both of these phosphorylated sphingoid bases in blood, accompanied by increases in very long chain ceramide species, and have defective lymphocyte trafficking. Surprisingly, levels of S1P and dihydro-S1P were increased in lymph from Spns2 mice as well as in specific tissues, including lymph nodes, and interstitial fluid. Moreover, lymph nodes from Spns2 mice have aberrant lymphatic sinus that appeared collapsed, with reduced numbers of lymphocytes. Our data suggest that Spns2 is an S1P transporter in vivo that plays a role in regulation not only of blood S1P but also lymph node and lymph S1P levels and consequently influences lymphocyte trafficking and lymphatic vessel network organization.
Figures
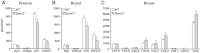

References
-
- Cyster J. G., Schwab S. R. (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 - PubMed
-
- Sato K., Malchinkhuu E., Horiuchi Y., Mogi C., Tomura H., Tosaka M., Yoshimoto Y., Kuwabara A., Okajima F. (2007) Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J. Neurochem. 103, 2610–2619 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32HL094290/HL/NHLBI NIH HHS/United States
- P30 NS047463/NS/NINDS NIH HHS/United States
- U19-AI077435/AI/NIAID NIH HHS/United States
- R01 CA160688/CA/NCI NIH HHS/United States
- R37-GM043880/GM/NIGMS NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- 5P30NS047463/NS/NINDS NIH HHS/United States
- T32 HL094290/HL/NHLBI NIH HHS/United States
- U19 AI077435/AI/NIAID NIH HHS/United States
- R01 GM043880/GM/NIGMS NIH HHS/United States
- P30CA16059/CA/NCI NIH HHS/United States
- K12 HD055881/HD/NICHD NIH HHS/United States
- K12-HD055881/HD/NICHD NIH HHS/United States
- R01 CA061774/CA/NCI NIH HHS/United States
- R37 GM043880/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

