DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function
- PMID: 23180828
- PMCID: PMC3597837
- DOI: 10.1189/jlb.0812394
DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function
Abstract
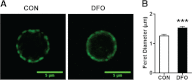
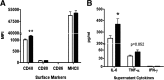
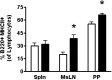
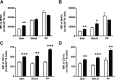
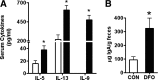
DHA is a n-3 LCPUFA in fish oil that generally suppresses T lymphocyte function. However, the effect of fish oil on B cell function remains relatively understudied. Given the important role of B cells in gut immunity and increasing human fish oil supplementation, we sought to determine whether DFO leads to enhanced B cell activation in the SMAD-/- colitis-prone mouse model, similar to that observed with C57BL/6 mice. This study tested the hypothesis that DHA from fish oil is incorporated into the B cell membrane to alter lipid microdomain clustering and enhance B cell function. Purified, splenic B cells from DFO-fed mice displayed increased DHA levels and diminished GM1 microdomain clustering. DFO enhanced LPS-induced B cell secretion of IL-6 and TNF-α and increased CD40 expression ex vivo compared with CON. Despite increased MHCII expression in the unstimulated ex vivo B cells from DFO-fed mice, we observed no difference in ex vivo OVA-FITC uptake in B cells from DFO or CON mice. In vivo, DFO increased lymphoid tissue B cell populations and surface markers of activation compared with CON. Finally, we investigated whether these ex vivo and in vivo observations were consistent with systemic changes. Indeed, DFO-fed mice had significantly higher plasma IL-5, IL-13, and IL-9 (Th2-biasing cytokines) and cecal IgA compared with CON. These results support the hypothesis and an emerging concept that fish oil enhances B cell function in vivo.
Figures

Comment in
-
Editorial: Fat chance to enhance B cell function.J Leukoc Biol. 2013 Apr;93(4):457-9. doi: 10.1189/jlb.1212646. J Leukoc Biol. 2013. PMID: 23547174 No abstract available.
Similar articles
-
Marine fish oils are not equivalent with respect to B-cell membrane organization and activation.J Nutr Biochem. 2015 Apr;26(4):369-77. doi: 10.1016/j.jnutbio.2014.11.005. Epub 2014 Dec 15. J Nutr Biochem. 2015. PMID: 25616447 Free PMC article.
-
Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis.Cancer Res. 2010 Oct 15;70(20):7960-9. doi: 10.1158/0008-5472.CAN-10-1396. Epub 2010 Aug 26. Cancer Res. 2010. PMID: 20798218
-
Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function.J Lipid Res. 2012 Apr;53(4):674-85. doi: 10.1194/jlr.M021782. Epub 2012 Feb 7. J Lipid Res. 2012. PMID: 22315394 Free PMC article.
-
Editorial: Fat chance to enhance B cell function.J Leukoc Biol. 2013 Apr;93(4):457-9. doi: 10.1189/jlb.1212646. J Leukoc Biol. 2013. PMID: 23547174 No abstract available.
-
N-3 fatty acids and membrane microdomains: from model membranes to lymphocyte function.Prostaglandins Leukot Essent Fatty Acids. 2012 Dec;87(6):205-8. doi: 10.1016/j.plefa.2012.09.007. Epub 2012 Oct 27. Prostaglandins Leukot Essent Fatty Acids. 2012. PMID: 23107229 Free PMC article. Review.
Cited by
-
Nutraceutical potential of Amazonian oilseeds in modulating the immune system against COVID-19 - A narrative review.J Funct Foods. 2022 Jul;94:105123. doi: 10.1016/j.jff.2022.105123. Epub 2022 May 24. J Funct Foods. 2022. PMID: 35634457 Free PMC article. Review.
-
Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance.Front Immunol. 2019 Jan 15;9:3160. doi: 10.3389/fimmu.2018.03160. eCollection 2018. Front Immunol. 2019. PMID: 30697214 Free PMC article. Review.
-
n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity.J Lipid Res. 2013 Nov;54(11):3130-8. doi: 10.1194/jlr.M042457. Epub 2013 Aug 28. J Lipid Res. 2013. PMID: 23986558 Free PMC article.
-
Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity.J Lipid Res. 2014 Jul;55(7):1420-33. doi: 10.1194/jlr.M049809. Epub 2014 May 17. J Lipid Res. 2014. PMID: 24837990 Free PMC article.
-
The Effects of a High-Fat Diet on Inflammatory Bowel Disease.Biomolecules. 2023 May 30;13(6):905. doi: 10.3390/biom13060905. Biomolecules. 2023. PMID: 37371485 Free PMC article. Review.
References
-
- Hermaszewski R. A., Webster A. D. B. (1993) Primary hypogammaglobulinemia—a survey of clinical manifestations and complications. Q. J. Med. 86, 31–42 - PubMed
-
- Harris D. P., Haynes L., Sayles P. C., Duso D. K., Eaton S. M., Lepak N. M., Johnson L. L., Swain S. L., Lund F. E. (2000) Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1, 475–482 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

