Dietary xenosterols lead to infertility and loss of abdominal adipose tissue in sterolin-deficient mice
- PMID: 23180829
- PMCID: PMC3588869
- DOI: 10.1194/jlr.M031476
Dietary xenosterols lead to infertility and loss of abdominal adipose tissue in sterolin-deficient mice
Abstract
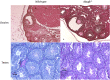
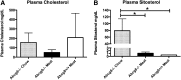
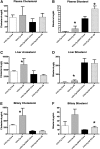
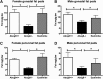
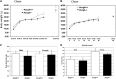
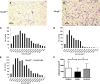
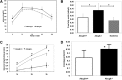
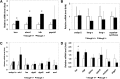
The investigation of the human disease sitosterolemia (MIM 210250) has shed light not only on the pathways by which dietary sterols may traffic but also on how the mammalian body rids itself of cholesterol and defends against xenosterols. Two genes, ABCG5 and ABCG8, located at the sitosterolemia locus, each encodes a membrane-bound ABC half-transporter and constitutes a functional unit whose activity has now been shown to account for biliary and intestinal sterol excretion. Knockout mice deficient in Abcg5 or Abcg8 recapitulate many of the phenotypic features of sitosterolemia. During the course of our studies to characterize these knockout mice, we noted that these mice, raised on normal rodent chow, exhibited infertility as well as loss of abdominal fat. We show that, although sitosterolemia does not lead to any structural defects or to any overt endocrine defects, fertility could be restored if xenosterols are specifically blocked from entry and that the loss of fat is also reversed by a variety of maneuvers that limit xenosterol accumulation. These studies show that xenosterols may have a significant biological impact on normal mammalian physiology and that the Abcg5 or Abcg8 knockout mouse model may prove useful in investigating the role of xenosterols on mammalian physiology.
Figures

Similar articles
-
Association of ABCG5 and ABCG8 Transporters with Sitosterolemia.Adv Exp Med Biol. 2024;1440:31-42. doi: 10.1007/978-3-031-43883-7_2. Adv Exp Med Biol. 2024. PMID: 38036873 Review.
-
A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol.BMC Med. 2004 Mar 24;2:5. doi: 10.1186/1741-7015-2-5. BMC Med. 2004. PMID: 15040800 Free PMC article.
-
Recent advances in understanding the STSL locus and ABCG5/ABCG8 biology.Curr Opin Lipidol. 2014 Jun;25(3):169-75. doi: 10.1097/MOL.0000000000000071. Curr Opin Lipidol. 2014. PMID: 24811295 Review.
-
The combination of ezetimibe and ursodiol promotes fecal sterol excretion and reveals a G5G8-independent pathway for cholesterol elimination.J Lipid Res. 2015 Apr;56(4):810-20. doi: 10.1194/jlr.M053454. Epub 2015 Jan 29. J Lipid Res. 2015. PMID: 25635125 Free PMC article.
-
Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of sitosterolemia.Blood. 2013 Oct 10;122(15):2732-42. doi: 10.1182/blood-2013-06-510461. Epub 2013 Aug 7. Blood. 2013. PMID: 23926302 Free PMC article.
Cited by
-
Association of ABCG5 and ABCG8 Transporters with Sitosterolemia.Adv Exp Med Biol. 2024;1440:31-42. doi: 10.1007/978-3-031-43883-7_2. Adv Exp Med Biol. 2024. PMID: 38036873 Review.
-
Case Report: Next Generation Sequencing in Clinical Practice-A Real Tool for Ending the Protracted Diagnostic Odyssey.Front Cardiovasc Med. 2022 Jan 13;8:778961. doi: 10.3389/fcvm.2021.778961. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35096999 Free PMC article.
-
Plant Sterols, Stanols, and Sitosterolemia.J AOAC Int. 2015 May-Jun;98(3):716-723. doi: 10.5740/jaoacint.SGEAjagbe. Epub 2015 May 4. J AOAC Int. 2015. PMID: 25941971 Free PMC article. Review.
-
Interferon regulatory factor 1 restricts gammaherpesvirus replication in primary immune cells.J Virol. 2014 Jun;88(12):6993-7004. doi: 10.1128/JVI.00638-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719409 Free PMC article.
-
Investigating Sitosterolemia to Understand Lipid Physiology.Clin Lipidol. 2013;8(3):649-658. doi: 10.2217/clp.13.60. Epub 2017 Jan 18. Clin Lipidol. 2013. PMID: 29928317 Free PMC article.
References
-
- Chiang J. Y. 2002. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr. Rev. 23: 443–463 - PubMed
-
- Kidambi S., Patel S. B. 2008. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: a review. Xenobiotica. 38: 1119–1139 - PubMed
-
- Lu K., Lee M. H., Patel S. B. 2001. Dietary cholesterol absorption; more than just bile. Trends Endocrinol. Metab. 12: 314–320 - PubMed
-
- Lee M-H., Gordon D., Ott J., Lu K., Ose L., Miettinen T., Gylling H., Stalenhoef A. F., Pandya A., Hidaka H., et al. 2001. Fine mapping of a gene responsible for regulating dietary cholesterol absorption; founder effects underlie cases of phytosterolemia in multiple communities. Eur. J. Hum. Genet. 9: 375–384 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

