Metallocenes as Target Specific Drugs for Cancer Treatment
- PMID: 23180884
- PMCID: PMC3501760
- DOI: 10.1016/j.ica.2012.06.007
Metallocenes as Target Specific Drugs for Cancer Treatment
Abstract
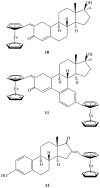
The application use of organometallic compounds into the cancer research was established in the late 1970s by Köpf-Maeir and Köpf. This new research area has been developed for the past thirty years. In the early 1980s, Jaouen and coworkers recognized the potential application of organometallic compounds vectorized with pendant groups that can deliver the drug to certain specific receptors. This is what is called nowdays Target Specific Drugs. This review will focus on metallocenes vectorized with steroids derivatives of hormones, nonsteroidal and selective endrocrine modulator.
Figures
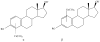
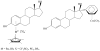
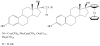
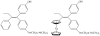
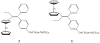



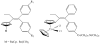



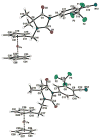
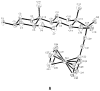
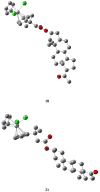


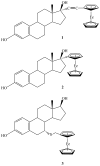
 are estradiol ferrocenoylate (of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate),
are estradiol ferrocenoylate (of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate),
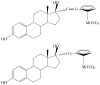
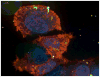
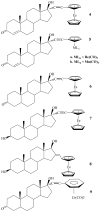
 is the nucleus.
is the nucleus.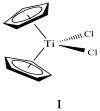




References
-
- Köpf H, Köpf-Maier P. Angew Chem Int Ed Engl. 1979;18:477–478. - PubMed
-
- Köpf-Maier P. Eur J Clin Pharmacol. 1994;47:1–16. - PubMed
-
- Köpf-Maier P, Köpf H. Structure and Bonding. 1988;70:103.
-
- Köpf-Maier P, Köpf H. Chem Rev. 1987;87:1137–1152.
-
- Köpf-Maier P, Köpf H. In: Metal Compounds in Cancer Therapy, Organometallic Titanium, Vanadium, Niobium, Molybdenum and Rhenium Complexes - Early Transition Metal Anti-tumor Drugs. Fricker SP, editor. Chapman and Hall; London: 1994. pp. 109–146.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
