Budget impact analysis of boceprevir and telaprevir for the treatment of hepatitis C genotype 1 infection
- PMID: 23180971
- PMCID: PMC3503472
- DOI: 10.2147/CEOR.S37205
Budget impact analysis of boceprevir and telaprevir for the treatment of hepatitis C genotype 1 infection
Abstract
Background: Boceprevir and telaprevir have recently showed dramatically better treatment outcomes than conventional PEGylated interferon plus ribavirin for the treatment of hepatitis C virus genotype 1, but the average cost per patient is unknown.
Methods: In the UK context, we performed a budget impact analysis to estimate the average per patient cost of adding boceprevir or telaprevir to PEGylated interferon plus ribavirin therapy. We considered both standard-duration therapy and response-guided therapy regimens of boceprevir and telaprevir for treatment-naïve and treatment-experienced patients. Our model utilized monthly discontinuation rates. We built a Bayesian Markov model to account for uncertainty associated with the clinical input and cost data.
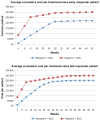
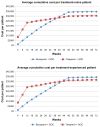
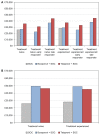
Results: The total average cost of response-guided therapy with boceprevir is £22,850 and £25,060 for treatment-naïve and treatment-experienced patients, respectively. By comparison, the total average cost of response-guided therapy with telaprevir was £29,930 and £31,880 for treatment-naïve and treatment-experienced patients, respectively, whereas the total average cost of standard-duration boceprevir is £34,680 and £34,350 and for telaprevir was £32,530 and £31,680 for treatment-naïve and treatment experienced patients, respectively.
Conclusion: Our results demonstrate that response-guided therapy with boceprevir is notably less costly than response-guided therapy with telaprevir. Our results also suggest that the standard treatment duration of boceprevir is slightly more costly than the standard treatment duration of telaprevir.
Keywords: boceprevir; budget impact analysis; hepatitis C; telaprevir.
Figures

References
-
- Poordad F, Khungar V. Emerging therapeutic options in hepatitis C virus infection. Am J Manag Care. 2011;17( Suppl 4):S123–S130. - PubMed
-
- Hofmann WP, Zeuzem S. A new standard of care for the treatment of chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2011;8:257–264. - PubMed
-
- Ciesek S, Manns MP. Hepatitis in 2010: the dawn of a new era in HCV therapy. Nat Rev Gastroenterol Hepatol. 2011;8:69–71. - PubMed
LinkOut - more resources
Full Text Sources

