Synthesis and antifungal activity of benzimidazole, benzotriazole and aminothiazole derivatives
- PMID: 23181082
- PMCID: PMC3501901
Synthesis and antifungal activity of benzimidazole, benzotriazole and aminothiazole derivatives
Abstract
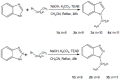
In recent years, the use of antifungal drugs in human medicine has increased, especially with the advent of AIDS epidemic. Efforts have focused on the development of new, less toxic and more efficacious antifungal drugs with novel mechanism of action. The purpose of this study was to synthesize of some new benzimidazole, benzotriazole and aminothiazole derivatives and to evaluate their activity against some species of Candida, Aspergillus and dermatophytes. The desired compounds were synthesized by the reaction of benzimidazole and benzotriazole with bromoalkanes and also by the reaction of an amide derivative of aminothiazole with 2-piperazino-1-ethanol in an efficient solvent in the presence of tetraethyl ammounim bromide or triethylamine) as catalyst. Chemical structures of all the new compounds were confirmed by spectrophotometric methods. Antifungal activities of the new compounds were evaluated by broth micro dilution method as recommended by CLSI. Among the tested compounds, 1-nonyl-1H-benzo[d]imidazole and 1-decyl-1H-benzo[d]imidazole exhibited the best antifungal activities. Of the examined synthetic compounds in different categories, benzimidazole derivatives established better antifungal activities than benzotriazole derivatives, and the piperazine analogue had no significant antifungal effect.
Keywords: Aminothiazole; Antifungal activity; Benzimidazole; Benzotriazole.
Figures
References
-
- Giraud F, Guillon R, Logé C, Pagniez F, Picot C, Borgne ML, et al. Synthesis and structure-activity relationships of 2-phenyl-1-[pyridinyl- and piperidinylmethyl) amino] -3-(1H-1,2,4-triazol-1-yl) propan-2-ols as antifungal agents. Bioorgan Med Chem Lett. 2009;19:301–304. - PubMed
-
- Masubuchi M, Ebiike H, Kawasaki KI, Sogabe S, Morikami K, Shiratori Y, et al. Synthesis and biological activities of benzofuran antifungal agents targeting fungal N-myristoyltransferase. Bioorgan Med Chem. 2003;11:4463–4478. - PubMed
-
- Andriole VT. Current and future antifungal therapy: New targets for antifungal therapy. Int J Antimicrob Ag. 2000;16:317–321. - PubMed
-
- De Pauw BE. New antifungal agents and preparations. Int J Antimicrob Ag. 2000;16:147–150. - PubMed
-
- Andriole VT. Current and future antifungal therapy: New targets for antifungal agents. J Antimicrob Chemoth. 1999;44:151–162. - PubMed
LinkOut - more resources
Full Text Sources