Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice
- PMID: 23181558
- PMCID: PMC3538623
- DOI: 10.1186/1743-7075-9-105
Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice
Abstract
Background: Type 2 diabetes is associated with obesity, ectopic lipid accumulation and low-grade inflammation. A dysfunctional gut microbiota has been suggested to participate in the pathogenesis of the disease. Green tea is rich in polyphenols and has previously been shown to exert beneficial metabolic effects. Lactobacillus plantarum has the ability to metabolize phenolic acids. The health promoting effect of whole green tea powder as a prebiotic compound has not been thoroughly investigated previously.
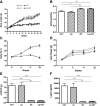
Methods: C57BL/6J mice were fed a high-fat diet with or without a supplement of 4% green tea powder (GT), and offered drinking water supplemented with Lactobacillus plantarum DSM 15313 (Lp) or the combination of both (Lp + GT) for 22 weeks. Parameters related to obesity, glucose tolerance, lipid metabolism, hepatic steatosis and inflammation were examined. Small intestinal tissue and caecal content were collected for bacterial analysis.
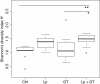
Results: Mice in the Lp + GT group had significantly more Lactobacillus and higher diversity of bacteria in the intestine compared to both mice in the control and the GT group. Green tea strongly reduced the body fat content and hepatic triacylglycerol and cholesterol accumulation. The reduction was negatively correlated to the amount of Akkermansia and/or the total amount of bacteria in the small intestine. Markers of inflammation were reduced in the Lp + GT group compared to control. PLS analysis of correlations between the microbiota and the metabolic variables of the individual mice showed that relatively few components of the microbiota had high impact on the correlation model.
Conclusions: Green tea powder in combination with a single strain of Lactobacillus plantarum was able to promote growth of Lactobacillus in the intestine and to attenuate high fat diet-induced inflammation. In addition, a component of the microbiota, Akkermansia, correlated negatively with several metabolic parameters known to be risk factors for the development of type 2 diabetes.
Figures


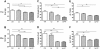

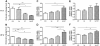


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

