From bortezomib to other inhibitors of the proteasome and beyond
- PMID: 23181572
- PMCID: PMC3657018
- DOI: 10.2174/1381612811319220012
From bortezomib to other inhibitors of the proteasome and beyond
Abstract
The cancer drug discovery field has placed much emphasis on the identification of novel and cancer-specific molecular targets. A rich source of such targets for the design of novel anti-tumor agents is the ubiqutin-proteasome system (UP-S), a tightly regulated, highly specific pathway responsible for the vast majority of protein turnover within the cell. Because of its critical role in almost all cell processes that ensure normal cellular function, its inhibition at one point in time was deemed non-specific and therefore not worth further investigation as a molecular drug target. However, today the proteasome is one of the most promising anti-cancer drug targets of the century. The discovery that tumor cells are in fact more sensitive to proteasome inhibitors than normal cells indeed paved the way for the design of its inhibitors. Such efforts have led to bortezomib, the first FDA approved proteasome inhibitor now used as a frontline treatment for newly diagnosed multiple myeloma (MM), relapsed/refractory MM and mantle cell lymphoma. Though successful in improving clinical outcomes for patients with hematological malignancies, relapse often occurs in those who initially responded to bortezomib. Therefore, the acquisition of bortezomib resistance is a major issue with its therapy. Furthermore, some neuro-toxicities have been associated with bortezomib treatment and its efficacy in solid tumors is lacking. These observations have encouraged researchers to pursue the next generation of proteasome inhibitors, which would ideally overcome bortezomib resistance, have reduced toxicities and a broader range of anti-cancer activity. This review summarizes the success and limitations of bortezomib, and describes recent advances in the field, including, and most notably, the most recent FDA approval of carfilzomib in July, 2012, a second generation proteasome inhibitor. Other proteasome inhibitors currently in clinical trials and those that are currently experimental grade will also be discussed.
Figures


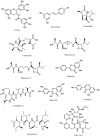
References
-
- Arrigo AP, Tanaka K, Goldberg AL, Welch WJ. Identity of the 19S `prosome' particle with the large multifunctional protease complex of mammalian cells (the proteasome) Nature. 1988;331(6152):192–4. - PubMed
-
- Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458(7237):438–44. - PubMed
-
- Groll M, Huber R, Moroder L. The persisting challenge of selective and specific proteasome inhibition. J Pept Sci. 2009;15(2):58–66. - PubMed
-
- Harding CV, France J, Song R, et al. Novel dipeptide aldehydes are proteasome inhibitors and block the MHC-I antigen-processing pathway. J Immunol. 1995;155(4):1767–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

