Quantum dot targeting with lipoic acid ligase and HaloTag for single-molecule imaging on living cells
- PMID: 23181687
- PMCID: PMC3528850
- DOI: 10.1021/nn304793z
Quantum dot targeting with lipoic acid ligase and HaloTag for single-molecule imaging on living cells
Abstract
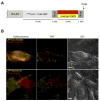
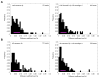
We present a methodology for targeting quantum dots to specific proteins on living cells in two steps. In the first step, Escherichia coli lipoic acid ligase (LplA) site-specifically attaches 10-bromodecanoic acid onto a 13 amino acid recognition sequence that is genetically fused to a protein of interest. In the second step, quantum dots derivatized with HaloTag, a modified haloalkane dehalogenase, react with the ligated bromodecanoic acid to form a covalent adduct. We found this targeting method to be specific, fast, and fully orthogonal to a previously reported and analogous quantum dot targeting method using E. coli biotin ligase and streptavidin. We used these two methods in combination for two-color quantum dot visualization of different proteins expressed on the same cell or on neighboring cells. Both methods were also used to track single molecules of neurexin, a synaptic adhesion protein, to measure its lateral diffusion in the presence of neuroligin, its trans-synaptic adhesion partner.
Figures


References
-
- Pinaud F, Clarke S, Sittner A, Dahan M. Probing Cellular Events, One Quantum Dot at a Time. Nat Methods. 2010;7:275–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

