Formylbenzene diazonium hexafluorophosphate reagent for tyrosine-selective modification of proteins and the introduction of a bioorthogonal aldehyde
- PMID: 23181702
- PMCID: PMC3526679
- DOI: 10.1021/bc300410p
Formylbenzene diazonium hexafluorophosphate reagent for tyrosine-selective modification of proteins and the introduction of a bioorthogonal aldehyde
Abstract
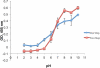
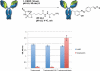
4-Formylbenzene diazonium hexafluorophosphate (FBDP) is a novel bench-stable crystalline diazonium salt that reacts selectively with tyrosine to install a bioorthogonal aldehyde functionality. Model studies with N-acyl-tyrosine methylamide allowed us to identify conditions optimal for tyrosine ligation reactions with small peptides and proteins. FBDP-based conjugation was used for the facile introduction of small molecule tags, poly(ethylene glycol) chains (PEGylation), and functional small molecules onto model proteins and to label the surface of living cells.
Figures


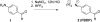
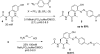


References
-
- Hooker JM, Kovacs EW, Francis MB. Interior surface modification of bacteriophage MS2. Journal of the American Chemical Society. 2004;126:3718–9. - PubMed
-
- Schlick TL, Ding Z, Kovacs EW, Francis MB. Dual-surface modification of the tobacco mosaic virus. Journal of the American Chemical Society. 2005;127:3718–23. - PubMed
-
- Jones MW, Mantovani G, Blindauer CA, Ryan SM, Wang X, Brayden DJ, Haddleton DM. Direct peptide bioconjugation/PEGylation at tyrosine with linear and branched polymeric diazonium salts. Journal of the American Chemical Society. 2012;134:7406–13. - PubMed
-
- Roglans A, Pla-Quintana A, Moreno-Manas M. Diazonium salts as substrates in palladium-catalyzed cross-coupling reactions. Chemical reviews. 2006;106:4622–43. - PubMed
-
- Hooker JM, Datta A, Botta M, Raymond KN, Francis MB. Magnetic resonance contrast agents from viral capsid shells: a comparison of exterior and interior cargo strategies. Nano letters. 2007;7:2207–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

