Characterization of the bipartite degron that regulates ubiquitin-independent degradation of thymidylate synthase
- PMID: 23181752
- PMCID: PMC3549573
- DOI: 10.1042/BSR20120112
Characterization of the bipartite degron that regulates ubiquitin-independent degradation of thymidylate synthase
Abstract
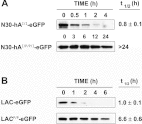
TS (thymidylate synthase) is a key enzyme in the de novo biosynthesis of dTMP, and is indispensable for DNA replication. Previous studies have shown that intracellular degradation of the human enzyme [hTS (human thymidylate synthase)] is mediated by the 26S proteasome, and occurs in a ubiquitin-independent manner. Degradation of hTS is governed by a degron that is located at the polypeptide's N-terminus that is capable of promoting the destabilization of heterologous proteins to which it is attached. The hTS degron is bipartite, consisting of two subdomains: an IDR (intrinsically disordered region) that is highly divergent among mammalian species, followed by a conserved amphipathic α-helix (designated hA). In the present report, we have characterized the structure and function of the hTS degron in more detail. We have conducted a bioinformatic analysis of interspecies sequence variation exhibited by the IDR, and find that its hypervariability is not due to diversifying (or positive) selection; rather, it has been subjected to purifying (or negative) selection, although the intensity of such selection is relaxed or weakened compared with that exerted on the rest of the molecule. In addition, we have verified that both subdomains of the hTS degron are required for full activity. Furthermore, their co-operation does not necessitate that they are juxtaposed, but is maintained when they are physically separated. Finally, we have identified a 'cryptic' degron at the C-terminus of hTS, which is activated by the N-terminal degron and appears to function only under certain circumstances; its role in TS metabolism is not known.
Figures







References
-
- Berger F. G., Berger S. H. Thymidylate synthase as a chemotherapeutic drug target: where are we after fifty years? Cancer Biol. Ther. 2006;5:1238–1241. - PubMed
-
- Carreras C. W., Santi D. V. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 1995;64:721–762. - PubMed
-
- Hardy L. W., Finer-Moore J. S., Montfort W. R., Jones M. O., Santi D. V., Stroud R. M. Atomic structure of thymidylate synthase: target for rational drug design. Science. 1987;235:448–455. - PubMed
-
- Phan J., Steadman D. J., Koli S., Ding W. C., Minor W., Dunlap R. B., Berger S. H., Lebioda L. Structure of human thymidylate synthase suggests advantages of chemotherapy with noncompetitive inhibitors. J. Biol. Chem. 2001;276:14170–14177. - PubMed
-
- Schiffer C. A., Clifton I. J., Davisson V. J., Santi D. V., Stroud R. M. Crystal structure of human thymidylate synthase: a structural mechanism for guiding substrates into the active site. Biochemistry. 1995;34:16279–16287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

