An oncolytic adenovirus regulated by a radiation-inducible promoter selectively mediates hSulf-1 gene expression and mutually reinforces antitumor activity of I131-metuximab in hepatocellular carcinoma
- PMID: 23182495
- PMCID: PMC5528471
- DOI: 10.1016/j.molonc.2012.10.007
An oncolytic adenovirus regulated by a radiation-inducible promoter selectively mediates hSulf-1 gene expression and mutually reinforces antitumor activity of I131-metuximab in hepatocellular carcinoma
Abstract
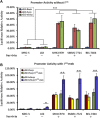
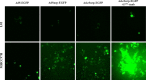
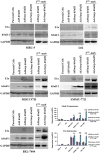
Gene therapy and antibody approaches are crucial auxiliary strategies for hepatocellular carcinoma (HCC) treatment. Previously, we established a survivin promoter-regulated oncolytic adenovirus that has inhibitory effect on HCC growth. The human sulfatase-1 (hSulf-1) gene can suppress the growth factor signaling pathways, then inhibit the proliferation of cancer cells and enhance cellular sensitivity to radiotherapy and chemotherapy. I(131)-metuximab (I(131)-mab) is a monoclonal anti-HCC antibody that conjugated to I(131) and specifically recognizes the HAb18G/CD147 antigen on HCC cells. To integrate the oncolytic adenovirus-based gene therapy and the I(131)-mab-based radioimmunotherapy, this study combined the CArG element of early growth response-l (Egr-l) gene with the survivin promoter to construct a radiation-inducible enhanced promoter, which was used to recombine a radiation-inducible oncolytic adenovirus as hSulf-1 gene vector. When I(131)-mab was incorporated into the treatment regimen, not only could the antibody produce radioimmunotherapeutic effect, but the I(131) radiation was able to further boost adenoviral proliferation. We demonstrated that the CArG-enhanced survivin promoter markedly improved the proliferative activity of the oncolytic adenovirus in HCC cells, thereby augmenting hSulf-1 expression and inducing cancer cell apoptosis. This novel strategy that involved multiple, synergistic mechanisms, including oncolytic therapy, gene therapy and radioimmunotherapy, was demonstrated to exert an excellent anti-cancer outcome, which will be a promising approach in HCC treatment.
Copyright © 2012 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Targeted Hsp70 expression combined with CIK-activated immune reconstruction synergistically exerts antitumor efficacy in patient-derived hepatocellular carcinoma xenograft mouse models.Oncotarget. 2015 Jan 20;6(2):1079-89. doi: 10.18632/oncotarget.2835. Oncotarget. 2015. PMID: 25473902 Free PMC article.
-
Augmenting the antitumor effect of TRAIL by SOCS3 with double-regulated replicating oncolytic adenovirus in hepatocellular carcinoma.Hum Gene Ther. 2011 Sep;22(9):1109-19. doi: 10.1089/hum.2010.219. Epub 2011 Apr 21. Hum Gene Ther. 2011. PMID: 21361790
-
A novel Golgi protein (GOLPH2)-regulated oncolytic adenovirus exhibits potent antitumor efficacy in hepatocellular carcinoma.Oncotarget. 2015 May 30;6(15):13564-78. doi: 10.18632/oncotarget.3769. Oncotarget. 2015. PMID: 25980438 Free PMC article.
-
Update on Application of Oncolytic Virus-Based Combination Therapy in the Treatment of Hepatocellular Carcinoma.J Biochem Mol Toxicol. 2025 Jun;39(6):e70327. doi: 10.1002/jbt.70327. J Biochem Mol Toxicol. 2025. PMID: 40488250 Review.
-
Tropism and transduction of oncolytic adenovirus vectors in prostate cancer therapy.Front Biosci (Landmark Ed). 2021 Oct 30;26(10):866-872. doi: 10.52586/4993. Front Biosci (Landmark Ed). 2021. PMID: 34719211 Review.
Cited by
-
Sulfatase 1 (hSulf-1) reverses basic fibroblast growth factor-stimulated signaling and inhibits growth of hepatocellular carcinoma in animal model.Oncotarget. 2014 Jul 15;5(13):5029-39. doi: 10.18632/oncotarget.2078. Oncotarget. 2014. PMID: 24970807 Free PMC article.
-
Targeted Hsp70 expression combined with CIK-activated immune reconstruction synergistically exerts antitumor efficacy in patient-derived hepatocellular carcinoma xenograft mouse models.Oncotarget. 2015 Jan 20;6(2):1079-89. doi: 10.18632/oncotarget.2835. Oncotarget. 2015. PMID: 25473902 Free PMC article.
-
Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma.Mol Cell Biochem. 2018 Jan;437(1-2):13-36. doi: 10.1007/s11010-017-3092-z. Epub 2017 Jun 7. Mol Cell Biochem. 2018. PMID: 28593566 Review.
-
Protein phosphatase PHLPP induces cell apoptosis and exerts anticancer activity by inhibiting Survivin phosphorylation and nuclear export in gallbladder cancer.Oncotarget. 2015 Aug 7;6(22):19148-62. doi: 10.18632/oncotarget.3721. Oncotarget. 2015. PMID: 25895131 Free PMC article.
-
Influential Factors and Synergies for Radiation-Gene Therapy on Cancer.Anal Cell Pathol (Amst). 2015;2015:313145. doi: 10.1155/2015/313145. Epub 2015 Dec 9. Anal Cell Pathol (Amst). 2015. PMID: 26783511 Free PMC article. Review.
References
-
- Banerjee, N.S. , Rivera, A.A. , Wang, M. , Chow, L.T. , Broker, T.R. , Curiel, D.T. , Nettelbeck, D.M. , 2004. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol. Cancer Ther. 3, 437–449. - PubMed
-
- Bickenbach, K.A. , Veerapong, J. , Shao, M.Y. , Mauceri, H.J. , Posner, M.C. , Kron, S.J. , Weichselbaum, R.R. , 2008. Resveratrol is an effective inducer of CArG-driven TNF-alpha gene therapy. Cancer Gene Ther. 15, 133–139. - PubMed
-
- Chen, Z.N. , Mi, L. , Xu, J. , Song, F. , Zhang, Q. , Zhang, Z. , Xing, J.L. , Bian, H.J. , Jiang, J.L. , Wang, X.H. , Shang, P. , Qian, A.R. , Zhang, S.H. , Li, L. , Li, Y. , Feng, Q. , Yu, X.L. , Feng, Y. , Yang, X.M. , Tian, R. , Wu, Z.B. , Leng, N. , Mo, T.S. , Kuang, A.R. , Tan, T.Z. , Li, Y.C. , Liang, D.R. , Lu, W.S. , Miao, J. , Xu, G.H. , Zhang, Z.H. , Nan, K.J. , Han, J. , Liu, Q.G. , Zhang, H.X. , Zhu, P. , 2006. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine 131I metuximab injection: clinical phase I/II trials. Int. J. Radiat. Oncol. Biol. Phys. 65, 435–444. - PubMed
-
- Chen, Z. , Fan, J.Q. , Li, J. , Li, Q.S. , Yan, Z. , Jia, X.K. , Liu, W.D. , Wei, L.J. , Zhang, F.Z. , Gao, H. , Xu, J.P. , Dong, X.M. , Dai, J. , Zhou, H.M. , 2009. Promoter hypermethylation correlates with the Hsulf-1 silencing in human breast and gastric cancer. Int. J. Cancer 124, 739–744. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

