The role of accessory proteins in the replication of feline infectious peritonitis virus in peripheral blood monocytes
- PMID: 23182908
- PMCID: PMC7117191
- DOI: 10.1016/j.vetmic.2012.10.032
The role of accessory proteins in the replication of feline infectious peritonitis virus in peripheral blood monocytes
Abstract
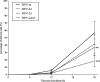
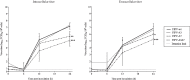
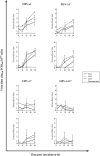
The ability to productively infect monocytes/macrophages is the most important difference between the low virulent feline enteric coronavirus (FECV) and the lethal feline infectious peritonitis virus (FIPV). In vitro, the replication of FECV in peripheral blood monocytes always drops after 12h post inoculation, while FIPV sustains its replication in the monocytes from 45% of the cats. The accessory proteins of feline coronaviruses have been speculated to play a prominent role in virulence as deletions were found to be associated with attenuated viruses. Still, no functions have been ascribed to them. In order to investigate if the accessory proteins of FIPV are important for sustaining its replication in monocytes, replication kinetics were determined for FIPV 79-1146 and its deletion mutants, lacking either accessory protein open reading frame 3abc (FIPV-Δ3), 7ab (FIPV-Δ7) or both (FIPV-Δ3Δ7). Results showed that the deletion mutants FIPV-Δ7 and FIPV-Δ3Δ7 could not maintain their replication, which was in sharp contrast to wt-FIPV. FIPV-Δ3 could still sustain its replication, but the percentage of infected monocytes was always lower compared to wt-FIPV. In conclusion, this study showed that ORF7 is crucial for FIPV replication in monocytes/macrophages, giving an explanation for its importance in vivo, its role in the development of FIP and its conservation in field strains. The effect of an ORF3 deletion was less pronounced, indicating only a supportive role of ORF3 encoded proteins during the infection of the in vivo target cell by FIPVs.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


References
-
- Addie D.D., Schaap I.A., Nicolson L., Jarrett O. Persistence and transmission of natural type I feline coronavirus infection. J. Gen. Virol. 2003;84:2735–2740. - PubMed
-
- Addie D.D., Toth S., Herrewegh A.A., Jarrett O. Feline coronavirus in the intestinal contents of cats with feline infectious peritonitis. Vet. Rec. 1996;139:522–530. - PubMed
-
- Chang H.W., de Groot R.J., Egberink H.F., Rottier P.J. Feline infectious peritonitis: insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J. Gen. Virol. 2010;91:415–420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

