Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496)
- PMID: 23182987
- PMCID: PMC3574266
- DOI: 10.1200/JCO.2012.43.4803
Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496)
Abstract
Purpose: Although ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) has been established as the standard of care in patients with advanced Hodgkin lymphoma, newer regimens have been investigated, which have appeared superior in early phase II studies. Our aim was to determine if failure-free survival was superior in patients treated with the Stanford V regimen compared with ABVD.
Patients and methods: The Eastern Cooperative Oncology Group, along with the Cancer and Leukemia Group B, the Southwest Oncology Group, and the Canadian NCIC Clinical Trials Group, conducted this randomized phase III trial in patients with advanced Hodgkin lymphoma. Stratification factors included extent of disease (localized v extensive) and International Prognostic Factors Project Score (0 to 2 v 3 to 7). The primary end point was failure-free survival (FFS), defined as the time from random assignment to progression, relapse, or death, whichever occurred first. Overall survival, a secondary end point, was measured from random assignment to death as a result of any cause. This design provided 87% power to detect a 33% reduction in FFS hazard rate, or a difference in 5-year FFS of 64% versus 74% at two-sided .05 significance level.
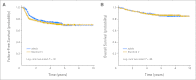
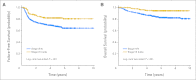
Results: There was no significant difference in the overall response rate between the two arms, with complete remission and clinical complete remission rates of 73% for ABVD and 69% for Stanford V. At a median follow-up of 6.4 years, there was no difference in FFS: 74% for ABVD and 71% for Stanford V at 5 years (P = .32).
Conclusion: ABVD remains the standard of care for patients with advanced Hodgkin lymphoma.
Trial registration: ClinicalTrials.gov NCT00003389.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



Comment in
-
Treatment of advanced Hodgkin lymphoma: the more things change, the more they stay the same.J Clin Oncol. 2013 Feb 20;31(6):660-2. doi: 10.1200/JCO.2012.44.7235. Epub 2012 Nov 26. J Clin Oncol. 2013. PMID: 23182992 No abstract available.
-
Treatment of advanced-stage hodgkin lymphoma: let us face the facts.J Clin Oncol. 2013 Aug 20;31(24):3045-6. doi: 10.1200/JCO.2013.49.1811. Epub 2013 Jul 8. J Clin Oncol. 2013. PMID: 23835702 No abstract available.
References
-
- Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin's lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–5396. - PubMed
-
- Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin's disease: Long-term results. J Clin Oncol. 2004;22:2835–2841. - PubMed
-
- Bonadonna G, Uslenghi C, Zucali R. Recent trends in the medical treatment of Hodgkin's disease. Eur J Cancer. 1975;11:251–266. - PubMed
-
- Bonadonna G, Zucali R, Monfardini S, et al. Combination chemotherapy of Hodgkin's disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–259. - PubMed
-
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478–1484. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA21076/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- CA13650/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- CA46441/CA/NCI NIH HHS/United States
- CA77202/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA77440/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- CA32102/CA/NCI NIH HHS/United States
- CA38926/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

