Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors
- PMID: 23183107
- PMCID: PMC3534778
- DOI: 10.1016/j.ejphar.2012.11.023
Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors
Abstract
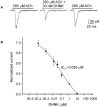
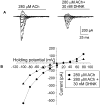
The effect of the (R,S)-ketamine metabolites (R,S)-norketamine, (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine on the activity of α7 and α3β4 neuronal nicotinic acetylcholine receptors was investigated using patch-clamp techniques. The data indicated that (R,S)-dehydronorketamine inhibited acetylcholine-evoked currents in α7-nicotinic acetylcholine receptor, IC(50) = 55 ± 6 nM, and that (2S,6S)-hydroxynorketamine, (2R,6R)-hydroxynorketamine and (R,S)-norketamine also inhibited α7-nicotinic acetylcholine receptor function at concentrations ≤ 1 μM, while (R,S)-ketamine was inactive at these concentrations. The inhibitory effect of (R,S)-dehydronorketamine was voltage-independent and the compound did not competitively displace selective α7-nicotinic acetylcholine receptor ligands [(125)I]-α-bungarotoxin and [(3)H]-epibatidine indicating that (R,S)-dehydronorketamine is a negative allosteric modulator of the α7-nicotinic acetylcholine receptor. (R,S)-Ketamine and (R,S)-norketamine inhibited (S)-nicotine-induced whole-cell currents in cells expressing α3β4-nicotinic acetylcholine receptor, IC(50) 3.1 and 9.1 μM, respectively, while (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine were weak inhibitors, IC(50) >100 μM. The binding affinities of (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine at the NMDA receptor were also determined using rat brain membranes and the selective NMDA receptor antagonist [(3)H]-MK-801. The calculated K(i) values were 38.95 μM for (S)-dehydronorketamine, 21.19 μM for (2S,6S)-hydroxynorketamine and>100 μM for (2R,6R)-hydroxynorketamine. The results suggest that the inhibitory activity of ketamine metabolites at the α7-nicotinic acetylcholine receptor may contribute to the clinical effect of the drug.
Published by Elsevier B.V.
Figures


References
-
- Adams JD, Jr, Baillie TA, Trevor AJ, Castagnoli N. Studies on the biotransformation of ketamine 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spec. 1981;8:527–538. - PubMed
-
- Bolze S, Boulieu R. HPLC determination of ketamine, norketamine and dehydronorketamine in plasma with a high-purity reversed-phase sorbent. Clin Chem. 1998;44:560–564. - PubMed
-
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333:99–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

