Protection of primary neurons and mouse brain from Alzheimer's pathology by molecular tweezers
- PMID: 23183235
- PMCID: PMC3525056
- DOI: 10.1093/brain/aws289
Protection of primary neurons and mouse brain from Alzheimer's pathology by molecular tweezers
Abstract
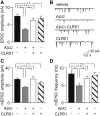
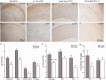
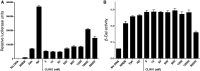
Alzheimer's disease is a devastating cureless neurodegenerative disorder affecting >35 million people worldwide. The disease is caused by toxic oligomers and aggregates of amyloid β protein and the microtubule-associated protein tau. Recently, the Lys-specific molecular tweezer CLR01 has been shown to inhibit aggregation and toxicity of multiple amyloidogenic proteins, including amyloid β protein and tau, by disrupting key interactions involved in the assembly process. Following up on these encouraging findings, here, we asked whether CLR01 could protect primary neurons from Alzheimer's disease-associated synaptotoxicity and reduce Alzheimer's disease-like pathology in vivo. Using cell culture and brain slices, we found that CLR01 effectively inhibited synaptotoxicity induced by the 42-residue isoform of amyloid β protein, including ∼80% inhibition of changes in dendritic spines density and long-term potentiation and complete inhibition of changes in basal synaptic activity. Using a radiolabelled version of the compound, we found that CLR01 crossed the mouse blood-brain barrier at ∼2% of blood levels. Treatment of 15-month-old triple-transgenic mice for 1 month with CLR01 resulted in a decrease in brain amyloid β protein aggregates, hyperphosphorylated tau and microglia load as observed by immunohistochemistry. Importantly, no signs of toxicity were observed in the treated mice, and CLR01 treatment did not affect the amyloidogenic processing of amyloid β protein precursor. Examining induction or inhibition of the cytochrome P450 metabolism system by CLR01 revealed minimal interaction. Together, these data suggest that CLR01 is safe for use at concentrations well above those showing efficacy in mice. The efficacy and toxicity results support a process-specific mechanism of action of molecular tweezers and suggest that these are promising compounds for developing disease-modifying therapy for Alzheimer's disease and related disorders.
Figures


References
-
- Thies W, Bleiler L Alzheimer’s Association 2011. Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–44. - PubMed
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Intl. 2004;11:36–42.
-
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimers amyloid β-protein by microglia in culture. J Neurosci Res. 1996;43:190–202. - PubMed
-
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

