Probing the structural basis for differential KCNQ1 modulation by KCNE1 and KCNE2
- PMID: 23183700
- PMCID: PMC3514736
- DOI: 10.1085/jgp.201210847
Probing the structural basis for differential KCNQ1 modulation by KCNE1 and KCNE2
Abstract
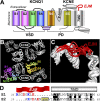
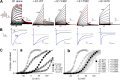
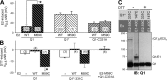
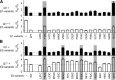
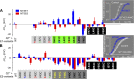
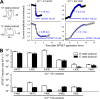
KCNE1 associates with KCNQ1 to increase its current amplitude and slow the activation gating process, creating the slow delayed rectifier channel that functions as a "repolarization reserve" in human heart. The transmembrane domain (TMD) of KCNE1 plays a key role in modulating KCNQ1 pore conductance and gating kinetics, and the extracellular juxtamembrane (EJM) region plays a modulatory role by interacting with the extracellular surface of KCNQ1. KCNE2 is also expressed in human heart and can associate with KCNQ1 to suppress its current amplitude and slow the deactivation gating process. KCNE1 and KCNE2 share the transmembrane topology and a high degree of sequence homology in TMD and surrounding regions. The structural basis for their distinctly different effects on KCNQ1 is not clear. To address this question, we apply cysteine (Cys) scanning mutagenesis to TMDs and EJMs of KCNE1 and KCNE2. We analyze the patterns of functional perturbation to identify high impact positions, and probe disulfide formation between engineered Cys side chains on KCNE subunits and native Cys on KCNQ1. We also use methanethiosulfonate reagents to probe the relationship between EJMs of KCNE subunits and KCNQ1. Our data suggest that the TMDs of both KCNE subunits are at about the same location but interact differently with KCNQ1. In particular, the much closer contact of KCNE2 TMD with KCNQ1, relative to that of KCNE1, is expected to impact the allosteric modulation of KCNQ1 pore conductance and may explain their differential effects on the KCNQ1 current amplitude. KCNE1 and KCNE2 also differ in the relationship between their EJMs and KCNQ1. Although the EJM of KCNE1 makes intimate contacts with KCNQ1, there appears to be a crevice between KCNQ1 and KCNE2. This putative crevice may perturb the electrical field around the voltage-sensing domain of KCNQ1, contributing to the differential effects of KCNE2 versus KCNE1 on KCNQ1 gating kinetics.
Figures





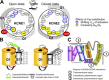
Similar articles
-
Probing the interaction between KCNE2 and KCNQ1 in their transmembrane regions.J Membr Biol. 2007 Apr;216(2-3):117-27. doi: 10.1007/s00232-007-9047-7. Epub 2007 Aug 4. J Membr Biol. 2007. PMID: 17676362
-
Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2.J Biol Chem. 2009 Jun 12;284(24):16452-16462. doi: 10.1074/jbc.M808262200. Epub 2009 Apr 16. J Biol Chem. 2009. PMID: 19372218 Free PMC article.
-
KCNE2 is colocalized with KCNQ1 and KCNE1 in cardiac myocytes and may function as a negative modulator of I(Ks) current amplitude in the heart.Heart Rhythm. 2006 Dec;3(12):1469-80. doi: 10.1016/j.hrthm.2006.08.019. Epub 2006 Aug 25. Heart Rhythm. 2006. PMID: 17161791
-
Structural basis for K(V)7.1-KCNE(x) interactions in the I(Ks) channel complex.Heart Rhythm. 2010 May;7(5):708-13. doi: 10.1016/j.hrthm.2009.12.017. Epub 2009 Dec 24. Heart Rhythm. 2010. PMID: 20206317 Free PMC article. Review.
-
The membrane protein KCNQ1 potassium ion channel: Functional diversity and current structural insights.Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183148. doi: 10.1016/j.bbamem.2019.183148. Epub 2019 Dec 9. Biochim Biophys Acta Biomembr. 2020. PMID: 31825788 Free PMC article. Review.
Cited by
-
Upgraded molecular models of the human KCNQ1 potassium channel.PLoS One. 2019 Sep 13;14(9):e0220415. doi: 10.1371/journal.pone.0220415. eCollection 2019. PLoS One. 2019. PMID: 31518351 Free PMC article.
-
Mechanism of external K+ sensitivity of KCNQ1 channels.J Gen Physiol. 2023 May 1;155(5):e202213205. doi: 10.1085/jgp.202213205. Epub 2023 Feb 21. J Gen Physiol. 2023. PMID: 36809486 Free PMC article.
-
Identification of KCNE6, a new member of the KCNE family of potassium channel auxiliary subunits.Commun Biol. 2024 Dec 19;7(1):1662. doi: 10.1038/s42003-024-07352-6. Commun Biol. 2024. PMID: 39702752 Free PMC article.
-
Allosteric mechanism for KCNE1 modulation of KCNQ1 potassium channel activation.Elife. 2020 Oct 23;9:e57680. doi: 10.7554/eLife.57680. Elife. 2020. PMID: 33095155 Free PMC article.
-
Photo-Cross-Linking of IKs Demonstrates State-Dependent Interactions between KCNE1 and KCNQ1.Biophys J. 2017 Jul 25;113(2):415-425. doi: 10.1016/j.bpj.2017.06.005. Biophys J. 2017. PMID: 28746852 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

