Carboxypeptidase M augments kinin B1 receptor signaling by conformational crosstalk and enhances endothelial nitric oxide output
- PMID: 23183746
- PMCID: PMC3566571
- DOI: 10.1515/hsz-2012-0290
Carboxypeptidase M augments kinin B1 receptor signaling by conformational crosstalk and enhances endothelial nitric oxide output
Abstract
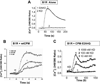
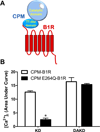
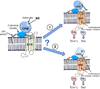
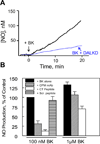
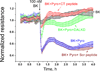
The G protein-coupled receptors (GPCRs) are the largest class of membrane proteins that play key roles in transducing extracellular signals to intracellular proteins to generate cellular responses. The kinin GPCRs, named B1 (B1R) and B2 (B2R), are responsible for mediating the biological responses to kinin peptides released from the precursor kininogens. Bradykinin (BK) or kallidin (KD) are agonists for B2Rs, whereas their carboxypeptidase (CP)-generated metabolites, des-Arg(9)-BK or des-Arg(10)-KD, are specific agonists for B1Rs. Here, we review the evidence for a critical role of membrane-bound CPM in facilitating B1R signaling by its ability to directly activate the receptor via conformational crosstalk as well as generate its specific agonist. In endothelial cells, the CPM/B1R interaction facilitates B1R-dependent high-output nitric oxide under inflammatory conditions.
Figures



Similar articles
-
Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling.J Biol Chem. 2011 May 27;286(21):18547-61. doi: 10.1074/jbc.M110.214940. Epub 2011 Mar 31. J Biol Chem. 2011. PMID: 21454694 Free PMC article.
-
Carboxypeptidase M and kinin B1 receptors interact to facilitate efficient b1 signaling from B2 agonists.J Biol Chem. 2008 Mar 21;283(12):7994-8004. doi: 10.1074/jbc.M709837200. Epub 2008 Jan 10. J Biol Chem. 2008. PMID: 18187413
-
Carboxypeptidase M is a positive allosteric modulator of the kinin B1 receptor.J Biol Chem. 2013 Nov 15;288(46):33226-40. doi: 10.1074/jbc.M113.520791. Epub 2013 Oct 9. J Biol Chem. 2013. PMID: 24108126 Free PMC article.
-
Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors.Neuropeptides. 2010 Apr;44(2):145-54. doi: 10.1016/j.npep.2009.12.004. Epub 2010 Jan 4. Neuropeptides. 2010. PMID: 20045558 Free PMC article. Review.
-
Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function.Hypertension. 2010 Feb;55(2):214-20. doi: 10.1161/HYPERTENSIONAHA.109.144600. Epub 2010 Jan 11. Hypertension. 2010. PMID: 20065150 Free PMC article. Review.
Cited by
-
Kininase 1 As a Preclinical Therapeutic Target for Kinin B1 Receptor in Insulin Resistance.Front Pharmacol. 2017 Aug 2;8:509. doi: 10.3389/fphar.2017.00509. eCollection 2017. Front Pharmacol. 2017. PMID: 28824433 Free PMC article.
-
Contact System Activation in Plasma from Dengue Patients Might Harness Endothelial Virus Replication through the Signaling of Bradykinin Receptors.Pharmaceuticals (Basel). 2021 Jan 12;14(1):56. doi: 10.3390/ph14010056. Pharmaceuticals (Basel). 2021. PMID: 33445640 Free PMC article.
-
Angiotensin-Converting Enzyme Inhibitor Protects Against Cisplatin Nephrotoxicity by Modulating Kinin B1 Receptor Expression and Aminopeptidase P Activity in Mice.Front Mol Biosci. 2020 May 20;7:96. doi: 10.3389/fmolb.2020.00096. eCollection 2020. Front Mol Biosci. 2020. PMID: 32528973 Free PMC article.
-
The effect of kinin B1 receptor on chronic itching sensitization.Mol Pain. 2015 Nov 14;11:70. doi: 10.1186/s12990-015-0070-x. Mol Pain. 2015. PMID: 26576537 Free PMC article.
-
Potentiation of Paclitaxel-Induced Pain Syndrome in Mice by Angiotensin I Converting Enzyme Inhibition and Involvement of Kinins.Mol Neurobiol. 2017 Dec;54(10):7824-7837. doi: 10.1007/s12035-016-0275-7. Epub 2016 Nov 14. Mol Neurobiol. 2017. PMID: 27844290
References
-
- Carretero OA, Scicli AG. Local hormonal factors (intracrine, autocrine, and paracrine) in hypertension. Hypertension. 1991;18:I58–I69. - PubMed
-
- Deddish PA, Skidgel RA, Kriho VB, Li XY, Becker RP, Erdös EG. Carboxypeptidase M in Madin-Darby canine kidney cells. Evidence that carboxypeptidase M has a phosphatidylinositol glycan anchor. J. Biol. Chem. 1990;265:15083–15089. - PubMed
-
- Erdös EG. Kinins, the long march--a personal view. Cardiovasc. Res. 2002;54:485–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
