The role of phosphatases in the initiation of skeletal mineralization
- PMID: 23183786
- PMCID: PMC3594124
- DOI: 10.1007/s00223-012-9672-8
The role of phosphatases in the initiation of skeletal mineralization
Abstract
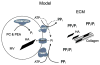
Endochondral ossification is a carefully orchestrated process mediated by promoters and inhibitors of mineralization. Phosphatases are implicated, but their identities and functions remain unclear. Mutations in the tissue-nonspecific alkaline phosphatase (TNAP) gene cause hypophosphatasia, a heritable form of rickets and osteomalacia, caused by an arrest in the propagation of hydroxyapatite (HA) crystals onto the collagenous extracellular matrix due to accumulation of extracellular inorganic pyrophosphate (PPi), a physiological TNAP substrate and a potent calcification inhibitor. However, TNAP knockout (Alpl(-/-)) mice are born with a mineralized skeleton and have HA crystals in their chondrocyte- and osteoblast-derived matrix vesicles (MVs). We have shown that PHOSPHO1, a soluble phosphatase with specificity for two molecules present in MVs, phosphoethanolamine and phosphocholine, is responsible for initiating HA crystal formation inside MVs and that PHOSPHO1 and TNAP have nonredundant functional roles during endochondral ossification. Double ablation of PHOSPHO1 and TNAP function leads to the complete absence of skeletal mineralization and perinatal lethality, despite normal systemic phosphate and calcium levels. This strongly suggests that the Pi needed for initiation of MV-mediated mineralization is produced locally in the perivesicular space. As both TNAP and nucleoside pyrophosphohydrolase-1 (NPP1) behave as potent ATPases and pyrophosphatases in the MV compartment, our current model of the mechanisms of skeletal mineralization implicate intravesicular PHOSPHO1 function and Pi influx into MVs in the initiation of mineralization and the functions of TNAP and NPP1 in the extravesicular progression of mineralization.
Conflict of interest statement
The author has stated that there is no conflict of interest.
Figures

References
-
- Glimcher MJ. Bone: nature of the calcium phosphate crystals and cellular, structural, and physical chemical mechanisms in their formation. Rev Mineral Geochem. 2006;64:223–282.
-
- Register TC, McLean FM, Low MG, Wuthier RE. Roles of alkaline phosphatase and labile internal mineral in matrix vesicle-mediated calcification. Effect of selective release of membrane-bound alkaline phosphatase and treatment with isosmotic pH 6 buffer. J Biol Chem. 1986;261:9354–9360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

